Chemistry PPT - WordPress.com
advertisement

THERMODYNAMICS
• Many chemicals reactions involve the
generation of gases capable of doing
mechanical work or the generation of heat.
• It is important to quantify these changes
and relate them to the changes in the
internal energy.
System, surroundings and boundary
Example: an open cup of coffee
(Closed system)
• Closed system – only energy can cross the
selected boundary
• Examples: a tightly capped cup of coffee
(isolated system)
• Isolated system – neither mass nor energy
can cross the selected boundary
• Example (approximate): coffee in a closed,
well-insulated thermos bottle
State Functions
• A state function is a property whose value
does not depend on the path taken to
reach that specific value. In contrast,
functions that depend on the path from
two values are call path functions. Both
path and state functions are often
encountered in thermodynamics.
• Whenever compounds or chemical
reactions are discussed, one of the first
things mentioned is the state of the specific
molecule or compound. "State" refers to
temperature, pressure, and the amount and
type of substance present. Once the state
has been established, state functions can be
defined.
• State functions are values that depend on the
state of the substance, and not on how that
state was reached. For example, density is a
state function, because a substance's density is
not affected by how the substance is obtained.
Consider a quantity of H2O: it does not
matter whether that H2O is obtained from
the tap, from a well, or from a bottle, because
as long as all three are in the same state, they
have the same density.
• When deciding whether a certain
property is a state function or not, keep
this rule in mind: is this property or
value affected by the path or way taken
to establish it? If the answer is no, then
it is a state function, but if the answer is
yes, then it is not a state function.
• Another way to think of state functions is as
integrals. Integrals depend on only three
things: the function, the lower limit and the
upper limit. Similarly, state functions depend
on three things: the property, the initial
value, and the final value. In other words,
integrals illustrate how state functions depend
only on the final and initial value and not on
the object's history or the path taken to get
from the initial to the final value.
• State functions are defined by comparing
them to path functions. As stated before, a
state function is a property whose value
does not depend on the path taken to
reach that specific function or value. In
essence, if something is not a path
function, it is probably a state function. To
better understand state functions, first
define path functions and then compare
path and state functions.
• In thermodynamics, the internal energy is
one of the two cardinal state functions of
the state variables of a thermodynamic
system. It refers to energy contained within
the system, while excluding the kinetic
energy of motion of the system as a whole
and the potential energy of the system as a
whole due to external force fields. It keeps
account of the gains and losses of energy
of the system.
• The internal energy of a system can be changed
by heating the system, or by doing work on it,
or by adding or taking away matter. When
matter transfer is prevented by impermeable
walls containing the system, it is said to be
closed. Then the first law of thermodynamics
states that the increase in internal energy is
equal to the total heat added and work done
on the system by the surroundings. If the
containing walls pass neither matter nor energy,
the system is said to be isolated. Then its
internal energy cannot change.
• The internal energy of a given state of a
system cannot be directly measured. It is
determined through some convenient chain of
thermodynamic operations and
thermodynamic processes by which the given
state can be prepared, starting with a
reference state which is customarily assigned a
reference value for its internal energy. Such a
chain, or path, can be theoretically described
by certain extensive state variables of the
system, namely, its entropy, S, its volume, V,
and its mole numbers, {Nj}. The internal
energy, U(S,V,{Nj}), is a function of those.
Sometimes, to that list are appended other
extensive state variables, for example electric
dipole moment.
• In thermodynamics, work performed by a
system is the energy transferred by the
system to another that is accounted for by
changes in the external generalized
mechanical constraints on the system. As
such, thermodynamic work is a
generalization of the concept of
mechanical work in physics.
• The external generalized mechanical constraints
may be chemical, electromagnetic, (including
radiative), gravitational or pressure/volume or
other simply mechanical constraints, including
momental, as in radiative transfer.
Thermodynamic work is defined to be
measurable solely from knowledge of such
external macroscopic constraint variables.
These macroscopic variables always occur in
conjugate pairs, for example pressure and
volume, magnetic flux density and
magnetization, mole fraction and chemical
potential. In the SI system of measurement,
work is measured in joules (symbol: J). The
rate at which work is performed is power.
• It is customary to calculate amount of energy
transferred as work through quantities external to
the system of interest, and thus belonging to its
surroundings. Nevertheless, for historical reasons,
the customary sign convention is to consider work
done by the system on its surroundings as positive.
Although all real physical processes entail some
dissipation of kinetic energy, it is matter of
principle that the dissipation that results from
transfer of energy as work occurs only inside the
system; energy dissipated outside the system, in the
process of transfer of energy, is not counted as
thermodynamic work. Thermodynamic work does
not account for any energy transferred between
systems as heat.
• Mechanical thermodynamic work is performed by
actions such as compression, and including shaft work,
stirring, and rubbing. In the simplest case, for example,
there are work of change of volume against a resisting
pressure, and work without change of volume, known
as isochoric work. An example of isochoric work is
when an outside agency, in the surrounds of the
system, drives a frictional action on the surface of the
system. In this case the dissipation is not necessarily
actually confined to the system, and the quantity of
energy so transferred as work must be estimated
through the overall change of state of the system as
measured by both its mechanically and externally
measurable deformation variables (such as its volume),
and its non-deformation variable (usually internal to
the system, for example its empirical temperature,
regarded not as a temperature but simply as a
mechanically measurable variable).
•
In a process of transfer of energy by work, the internal
energy of the final state of the system is then measured
by the amount of adiabatic work of change of volume
that would have been necessary to reach it from the
initial state, such adiabatic work being measurable only
through the externally measurable mechanical or
deformation variables of the system, but including also
full information about the forces exerted by the
surroundings on the system during the process. In the
case of some of Joule's measurements, the process was
so arranged that heat produced outside the system by
the frictional process was practically entirely transferred
into the system during the process, so that the quantity
of work done by the surrounds on the system could be
calculated as shaft work, an external mechanical
variable. For closed systems, internal energy changes in
a system other than as work transfer are as heat.
• In physics, heat is the transfer of energy other
than by work or transfer of matter. Heat
flows spontaneously from a hotter body to a
colder one whenever a suitable physical
pathway exists between the bodies, and
always results in a net increase in entropy. The
pathway can be direct, as in conduction and
radiation, or indirect, as in convective
circulation. Because it refers to a process, heat
is not a property of a system.
• Kinetic theory explains heat as a macroscopic
manifestation of the motions and interactions
of microscopic constituents such as molecules
and photons.The quantity of energy
transferred as heat is a scalar expressed in an
energy unit such as the joule (J) (SI), with a
sign that is customarily positive when a
transfer adds to the energy of a system. It can
be measured by calorimetry,[10] or
determined by calculations based on other
quantities, relying on the first law of
thermodynamics. In calorimetry, latent heat
changes a system's state without temperature
change, while sensible heat changes its
temperature.
• If latent heat is defined with respect to a
change of a particular state variable of the
system, then a specifically corresponding
variety of constrained sensible heat can be
defined for change of temperature, leaving
that particular state variable unchanged.
For infinitesimal changes, the total
incremental heat transfer is then the sum of
the latent and sensible heat increments.
This is a basic paradigm for
thermodynamics, and was important in the
historical development of the subject.
• Referring to conduction, Partington writes:
"If a hot body is brought in conducting
contact with a cold body, the temperature
of the hot body falls and that of the cold
body rises, and it is said that a quantity of
heat has passed from the hot body to the
cold body.“ Referring to radiation,
Maxwell writes: "In Radiation, the hotter
body loses heat, and the colder body
receives heat by means of a process
occurring in some intervening medium
which does not itself thereby become hot."
• Maxwell writes that convection as such "is
not a purely thermal phenomenon". In
thermodynamics, convection in general is
regarded as transport of internal energy. If,
however, the convection is enclosed and
circulatory, then it may be regarded as an
intermediary that transfers energy as heat
between source and destination bodies,
because it transfers only energy and not
matter from the source to the destination
body.
• An adiabatic process is one that occurs without
transfer of heat or matter between a system and its
surroundings. A key concept in thermodynamics,
the adiabatic process provides a rigorous
conceptual basis for the theory used to expound
the first law of thermodynamics. For some practical
and theoretical purposes, some chemical and
physical processes occur so rapidly that they can be
conveniently described as an "adiabatic
approximation", meaning that there is hardly time
for transfer of energy as heat. Such processes are
often followed or preceded by processes that are
not adiabatic.
• A process that does not involve the transfer of
heat into or out of a system Q = 0, is called
an adiabatic process, and such a system is said
to be adiabatically isolated. The assumption
of an adiabatic process or isolation is
frequently made when analyzing a system
from the stand point of thermodynamics. For
example, the compression of the gas within a
cylinder of a diesel engine is assumed to occur
so rapidly such that on the time scale of the
compression process, little of the system's
energy can be transferred out as heat. Even
though the cylinders are not insulated and are
quite conductive, that process is idealized to
be adiabatic.
• The assumption of adiabatic isolation is a
useful one, and is often combined with
other assumptions about a system so as to
make the calculation of the system's
behavior possible. Such assumptions are
idealizations. The behavior of actual
machines deviates from these idealizations,
but the assumption of such "perfect"
behavior are useful first approximations
about how the real world works.
• The change in a system's internal energy is
equal to the difference between heat
added to the system from its surroundings
and work done by the system on its
surroundings.
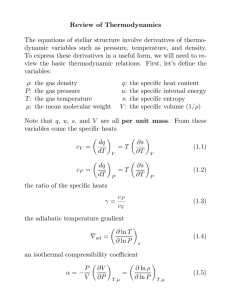
• Mathematical Representation of the First Law
• Physicists typically use uniform conventions for representing the
quantities in the first law of thermodynamics. They are:
U1 (or Ui) = initial internal energy at the start of the process
U2 (or Uf) = final internal energy at the end of the process
delta-U = U2 - U1 = Change in internal energy (used in cases where
the specifics of beginning and ending internal energies are irrelevant)
•
Q = heat transferred into (Q > 0) or out of (Q < 0) the system
•
W = work performed by the system (W > 0) or on the system (W
< 0).
•
•
•
• This yields a mathematical representation of the first law which proves
very useful and can be rewritten in a couple of useful ways:
•
U 2 - U 1 = delta- U = Q - W
•
Q = delta-U + W
Thank
You