Review of Thermodynamics The equations of stellar structure
advertisement

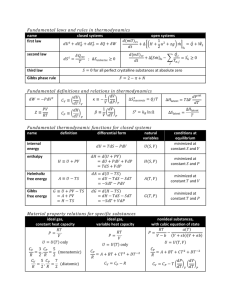
Review of Thermodynamics The equations of stellar structure involve derivatives of thermodynamic variables such as pressure, temperature, and density. To express these derivatives in a useful form, we will need to review the basic thermodynamic relations. First, let’s define the variables: ρ: P: T: µ: the the the the gas density gas pressure gas temperature mean molecular weight q: u: s: V: the the the the specific specific specific specific heat content internal energy entropy volume (1/ρ) Note that q, u, s, and V are all per unit mass. From these variables come the specific heats µ ¶ µ ¶ dq ∂s cV = =T (1.1) dT V ∂T V µ ¶ µ ¶ dq ∂s cP = =T (1.2) dT P ∂T P the ratio of the specific heats γ= cP cV the adiabatic temperature gradient ¶ µ ∂ ln T ∇ad = ∂ ln P s an isothermal compressibility coefficient µ ¶ µ ¶ P ∂V ∂ ln ρ α=− = V ∂P T,µ ∂ ln P T,µ (1.3) (1.4) (1.5) a volume coefficient of expansion T δ= V µ ∂V ∂T ¶ =− P,µ µ ∂ ln ρ ∂ ln T ¶ µ ∂ ln ρ ∂ ln µ ¶ (1.6) P,µ and a chemical potential coefficient µ ϕ=− V µ ∂V ∂µ ¶ = P,T (1.7) P,T (For the following, we will assume the chemical composition is fixed.) We will also need the first law of thermodynamics: dq = T ds = du + P dV (1.8) Note that although there are four variables in this equation (s, T , P , and V ), only two are independent. To derive the relationships between the various thermodynamic variables, first take s and V as independent, and re-write (1.8) as du = T ds − P dV (1.9) However, when written in terms of s and V , du is formally du = µ ∂u ∂s ¶ ds + V which means that µ ¶ ∂u =T ∂s V µ ∂u ∂V µ and ¶ ∂u ∂V dV s ¶ = −P (1.10) s Now, mathematically ∂2u ∂2u = ∂V ∂s ∂s∂V so µ ∂u ∂V ¶ µ s ∂u ∂s ¶ = V µ ∂u ∂s ¶ µ V ∂u ∂V ¶ s or µ ∂T ∂V ¶ =− s µ ∂P ∂s ¶ (1.11) V Similarly, if we choose s and P as the independent variables, and add d(P V ) to each side of (1.9), then the first law of thermodynamics becomes dH = d(u + P V ) = T ds − P dV + P dV + V dP = T ds + V dP The total first derivative of H is then ¶ µ ¶ µ ∂H ∂H ds + dP dH = ∂s P ∂P s which implies that µ ¶ ∂H =T ∂s P and µ ∂T ∂P µ and ¶ = s µ ∂V ∂s ∂H ∂P ¶ ¶ =V s (1.12) P If we subtract d(T s) from each side of the first law of thermodynamics, then T and V are the free parameters, via dF = d(u − T s) = T ds − P dV − T ds − sdT = −P dV − sdT We then get the relations µ ¶ ∂F = −s ∂T V and µ ∂F ∂V which leads via the second derivatives to ¶ µ ¶ µ ∂s ∂P = ∂V T ∂T V ¶ = −P T (1.13) Finally, if T and P are chosen to be independent, and d(P V −T s) are added to (1.8), then we can derive the relation µ ¶ µ ¶ ∂s ∂V =− (1.14) ∂P T ∂T P Thus, we have Maxwell’s relations µ ∂T ∂V µ µ ¶ ∂T ∂P =− s ¶ = s µ µ ∂P ∂s ∂V ∂s ¶ ¶ (1.11) V (1.12) P ¶ µ ¶ ∂s ∂P = ∂V T ∂T V µ ¶ µ ¶ ∂s ∂V =− ∂P T ∂T P (1.13) (1.14) To derive a relation between the specific heats, start by letting T and P be independent, and write the specific heat content as dq = T ds = T and dP as dP = µ ·µ ∂P ∂T ∂s ∂T ¶ ¶ dT + P dT + V µ µ ∂P ∂V ∂s ∂P ¶ ¶ dV dP T ¸ T This gives dq = T µ ∂s ∂T ¶ dT + T P µ ∂s ∂P ¶ ·µ T ∂P ∂T ¶ dT + V µ ∂P ∂V ¶ dV T ¸ We can now evaluate (dq/dT ) while holding V constant, i.e., with dV = 0 µ ¶ µ ¶ µ ¶ µ ¶ dq ∂s ∂s ∂P =T +T dT V ∂T P ∂P T ∂T V The term on the left side of the equation is cV , the first term on the right is cP , and (ds/dP )T = −(dV /dT )P by a Maxwell relation (1.14). Thus, cP − cV = T µ ∂V ∂T ¶ µ P ∂P ∂T ¶ V The first partial differential can immediately be written in terms of the volume coefficient of expansion (1.6) µ ∂V ∂T ¶ = P Vδ T (1.15) The second partial differential can also be re-written, if one first notes that the total derivative for dV is µ ¶ µ ¶ ∂V ∂V dV = dT + dP ∂T P ∂P T Thus, when V is held constant, dV = 0, and µ ∂P ∂T ¶ =− V µ ∂V ∂T ¶ Áµ P ∂V ∂P ¶ T The numerator on the right side is again (δ/ρT ), while the denominator is related to the compressibility coefficient by µ Thus and ∂V ∂P µ ¶ ∂P ∂T =− T ¶ = V αV P Pδ Tα P V δ2 P δ2 cP − cV = = Tα ρT α Note that this reduces to R = k/mA for an ideal gas. (1.16) (1.17) Finally, to express the change in the heat content of a system, dq, in terms of the intensive parameters only, choose V and T as the independent variables, and write the change in entropy as ¶ ¶ µ µ ∂s ∂s ds = dT + dV ∂T V ∂V T Using the definition of heat capacity (1.1) and the Maxwell relation (1.13), this becomes µ ¶ ∂P cV dT + dV ds = T ∂T V If we now substitude (1.16) for (∂P/∂T )V , and convert dV to dρ using dV = −1/ρ2 dρ, we get an expression for dq dq = T ds = cV dT − P δ dρ ρα ρ This can then be further simplified by noting that µ ¶ µ ¶ dρ ∂ ln ρ ∂ ln ρ dP dT = d ln P + d ln T = α −δ ρ ∂ ln P T ∂ ln T P P T Thus P δ2 δ dq = cV dT + dT − dP ρT α ρ or δ dq = cP dT − dP ρ (1.18) This equation also leads directly to an expression for the adiabatic temperature gradient. If dq = 0, then cP dT = δ dP ρ which implies that µ and ∇ad = ∂T ∂P µ ¶ = δ ρcP ¶ = s ∂ ln T ∂ ln P s Pδ T ρcP (1.19) Note that for an ideal gas, the definition of an adiabat implies that µ ¶γ P P ∝ ργ ∝ =⇒ T ∝ P (γ−1)/γ T Hence for a monotonic gas with γ = 5/3, ∇ad Á = (2/3) (5/3) = 0.4 (1.20) Note also that (1.19) can then be substituted back into (1.18) to yield an equation for dq in terms of P , T , cP , and adiabatic temperature gradient · ¸ dT dP T cP ∇ad dP = cP T − ∇ad dq = cP dT − P T P (1.21)