Water Treatment - Vos instrumenten
advertisement

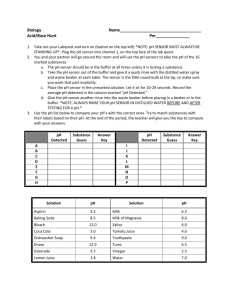
Water Treatment 012-10992 r1.04 Water Treatment 012-10758 r1.04 Water Treatment Introduction Journals and Snapshots The Snapshot button is used to capture the screen. The Journal is where snapshots are stored and viewed. The Share button is used to export or print your journal to turn in your work. Each page of this lab that contains the symbol should be inserted into your journal. After completing a lab page with the snapshot symbol, tap (in the upper right hand corner) to insert the page into your journal. Note: You may want to take a snapshot of the first page of this lab as a cover page for your journal. Water Treatment Lab Challenge • What are some of the processes used to treat water and make it safe to drink? What contaminants are removed by water treatment methods? • How would you design an effective water treatment system? Water Treatment Background • There are several steps in the water treatment process. A primary step is filtration. • Filtration technology is very effective and can remove nearly any impurities from water but it is not very cost-effective (replacement filters are costly). • So most water treatment systems use filtration in combination with other simpler methods like coagulation, flocculation, and chemical disinfection. Water Treatment Self-check 1. Why don't municipal water treatment plants simply use giant filters to clean water, similar to the portable filters used by hikers? a) Filters are not effective at cleaning water. b) Replacing large filters is too expensive. c) Giant filters are too heavy to carry while hiking. d) Filter technology is not reliable. Water Treatment ...Background • Both types of water treatment—for wastewater or sink/shower water—start by passing the water through a gridded screen to catch larger objects. • Then a coagulant is added to the water. Salts of aluminum (alum) or water-soluble organic polyelectrolytes are often used as coagulants. Coagulants cause suspended particles to form clumps. • These clumps aggregate into larger clumps, or flocs, during the flocculation process. The flocs are dense enough to settle out of the water in a process called sedimentation. Water Treatment Self-check 2. What is the purpose of a coagulant? Water Treatment ...Background • The water is then filtered through a variety of media of varying porosities, including activated carbon, sand, and gravel. Filtration works by trapping impure particles in the filtration media as the water slips through the cracks. • At this point the water looks clean but is still not quite ready. Several final disinfecting precautions must be taken to eliminate viral or bacterial sources that can cause disease. Three common ways to disinfect are treatment with chlorine, bubbling ozone through the water, or shining ultraviolet light through the water. Water Treatment ...Background Water from source Drinking water to homes Filtration tanks Coagulant addition Mixing/floccula tion basin Sedimentati on basin Sludge to disposal Storage reservoir Chlorination Water Treatment Self-check 3. Why is it necessary to further treat water once it has been through the filtering process? Water Treatment Safety • Follow all standard laboratory safety procedures. • Wear safety glasses. • Keep water away from sensitive electronic equipment. Water Treatment Materials and Equipment Collect all of these materials before beginning the lab. • Water quality sensor (or separate • Activated charcoal (2 g) pH and conductivity sensors) • Swimming pool water clarifier solution, • Turbidity sensor (optional) 4% (2-mL) • Beaker, 150-mL (4) • "Wastewater" sample (500-mL) • Beaker, 50-mL (1) • Tap water • Large beaker for containing waste • Wash bottle containing deionized water • Large test tube, 18-mm or more • Paper towels, kitchen, white, roll (several) • Pipette and bulb • Lint-free lab tissue • 500-mL soda bottle • Paper napkins, white, smooth (12) • Stirring rod Water Treatment Sequencing Challenge: Part 1 A. Connect the pH, conductivity, and turbidity sensors. Calibrate the turbidity sensor. B. Observe the odor and measure the pH, conductivity, and turbidity of the "wastewater" water sample. C. Make a paper filter and use it to filter the "wastewater" water sample. Make observations and measurements as before. D. Pre set-up sedimentation, agglutination, and "wastewater" samples. Set aside for at least 30 minutes. Set up your activated charcoal. The steps to the left are PART A of the procedure for this lab activity. They are not in the right order. Write the correct sequence below, then take a snapshot of this page. Water Treatment Sequencing Challenge: Part 2 A. Collect an aliquot of the "wastewater" sample. Observe its odor and measure the pH, conductivity, and turbidity. B. Make a paper filter; filter the agglutinated water sample. Make observations and measurements as before. C. Use an activated charcoal-coated filter on the "wastewater" water. Observe odor, color, pH, conductivity, and turbidity. D. Test the pH/conductivity of the charcoal-filtered water. E. Test your design and evaluate the results. F. Design a water treatment technique using the technologies you studied in this lab that you think will best treat the wastewater. The steps to the left are PART B of the procedure for this lab activity. They are not in the right order. Write the correct sequence below, then take a snapshot of this page. Water Treatment Setup 1. Stir the "wastewater" sample to uniformly mix it. 2. Pour 100 mL of the well-mixed "wastewater" sample into each of four 150-mL beakers. Label the beakers as follows: Beaker 1 = “Untreated” Beaker 2 = “Activated Charcoal” Beaker 3 = “Sedimentation” Beaker 4 = “Coagulation” (agglutination) 3. Set Beaker 3 (sedimentation) aside for at least 30 minutes. Water Treatment Setup 4. To set up the agglutination sample, put 2 mL of the 4% swimming pool clarifier solution into Beaker #4 and stir vigorously. Note: Swimming pool clarifier contains coagulating agents that are similar to those used in municipal water treatment facilities. 5. Record any changes in appearance below. Periodically stir this solution over the next 30 minutes. Water Treatment Setup 6. Create a membrane filter. Start by cutting off the bottom half of a plastic 500-mL soda bottle. Turn the top half over as a simple funnel. 7. Fold a paper towel in half, and then fold it in half again. Separate the layers to make a funnel. 8. Stack 3 paper napkins together, and shape them into a shallow bowl. Tuck these into the paper funnel, and push the entire membrane construction into the funnel, forming a bowl to hold the filtrant. Set this aside for now. Water Treatment Setup 9. Connect a water quality sensor (or pH and conductivity sensors) to your data collection system. Note: It is not necessary to calibrate the conductivity sensor for this activity. 10. Calibrate the pH sensor (see instructions in blue on next page). 11. Using an extension cable, connect and calibrate the turbidity sensor using the two calibration samples provided with your sensor. For each test, you will be taking an aliquot (small sample) with the clean sample bottles provided with your turbidity sensor. Note: The use of the turbidity sensor in this lab is optional. If you are not using a turbidity sensor, skip procedures that use this sensor. Water Treatment To Calibrate the pH Sensor: Note: Only calibrate the sensor if 3. Calibration Point 1: instructed to do so by your teacher. a. Place the pH probe in a pH 4 buffer solution. Note: During the calibration process you b. Enter 4.0 as the pH in the Standard Value box will not be able to return to this page. under Calibration Point 1. c. Tap Read From Sensor under Calibration Point 1. Open the Calibrate Sensor screens: 1. a. Tap d. Rinse the pH probe thoroughly using distilled b. Tap CALIBRATE SENSOR water. 2. Ensure that the correct measurements are selected: a. Sensor: (name of sensor) Measurement: pH Calibration Type: 2 point b. Tap NEXT 4. Calibration Point 2: a. Repeat the process used in calibration point 1 using a pH 10 buffer solution. b. Tap OK to exit the calibration screen and then tap OK again to return to the lab. Water Treatment Predict Q1: Which water treatment method is most likely to remove odors, colors, particles, etc? Make predictions in the space below. Possible methods include Metal Gratings, Coagulation, Flocculation, Sedimentation, Filtration, and Disinfection. Water Treatment Procedure 1. Examine the untreated “wastewater” in Beaker #1. After you begin to collect data, you will be able to record your observations in a data table that will expand as you examine all your samples. 2. Make a note of the odor, color and appearance of the wastewater in Beaker #1. You will record this in the table on pg. 23. Water Treatment 3. Measure the untreated water with the pH sensor. 6. Next measure the untreated water with the conductivity sensor. 8. Then take an aliquot of the untreated water and measure with the turbidity sensor. 4. Tap to activate the sensor and to deactivate. 7. Record the result in the data table on next page. 9. Record the result in the data 5. Record the result in the data table on next page. table on next page. Water Treatment *To Enter Data into a Table: 1. Tap to open the tool palette. 2. Tap then tap a cell in the data table to highlight it in yellow. 3. Tap to open the Keyboard screen. Water Treatment 10.Now filter this untreated wastewater by pouring half of it into the paper filter. Collect the filtered wastewater (filtrate) in a 50-mL beaker. Be careful to keep the liquid contained inside the paper napkin "bowl". Don't let it overflow! 11. Record the odor an color/appearance of the filtered water in the adjacent data table. Water Treatment 12. Transfer the filtered water 15. Next measure the filtrate (filtrate) to a large test with the conductivity tube. sensor. 13. Measure the filtrate with 16. Record the result on the the pH sensor. next page. 14. Record the result in the data table on the next page. 17. Finally, take an aliquot and measure the filtrate with the turbidity sensor. 18. Record the result on the next page. 19. Rinse all beakers and test tubes. Water Treatment Water Treatment Procedure: Membrane Filtration Did your simple filter turn the "wastewater" into clear drinking water? Probably not! So let's look at some additional water treatment methods on the next page. Water Treatment Procedure: Membrane Filtration + Activated Charcoal 1. Create a membrane filter with activated charcoal. Reuse the plastic bottle funnel. Fold a paper towel in half, and then fold it in half again. Separate the layers to make a funnel. Stack 3 paper napkins into a shallow bowl and tuck these into the paper funnel. Push the entire membrane construction into the funnel, forming a bowl to hold the filtrant. 2. Add 1 gram of activated charcoal to 100 mL of tap water and stir. Put this slurry into the filter. Water Treatment Procedure: Membrane Filtration + Activated Charcoal 3. Slowly pour an additional 100 mL of tap water into the filter. You should now have a membrane filter covered with a layer of activated charcoal. If the tap water filtering through it is not clear, filter an additional 100 mL of tap water. Note: This step ensures that the activated carbon becomes fixed inside the filter and is not leaking out into the filtrate. Note: Activated charcoal is prepared so that it is extremely porous and can thus trap and remove molecules, especially large organic molecules such as those responsible for odors and colors. The activated charcoal must then be filtered out of the sample. Water Treatment 4. Pour about 80 mL of the "wastewater" from Beaker #2 into the charcoal filter. 5. As the water filters through, discard the first 30 mL or so. Collect the remaining filtrate in a clean 50 mL beaker. 6. Examine the resulting filtrate. Record its odor, color and appearance in the adjacent data table. 7. Transfer the filtrate to a large test tube for further sensor testing. Water Treatment 8. Measure the filtrate in the large test tube with the pH sensor. 9. Tap to activate the sensor and to deactivate. 10. Record result in data table on next page. 11. Next measure the filtrate with the conductivity sensor. 12. Record the result in the data table on next page. 13. Finally, measure the filtrate with the turbidity sensor. 14. Record result in the data table on next page. 15. Rinse beakers and test tube. Water Treatment Water Treatment Procedure: Coagulation + Filter 1. Examine the coagulated sample in Beaker #4. 2. Note any major differences in appearance between the untreated wastewater (Beaker #1) and the coagulated wastewater (Beaker #4). Record your observations below. Water Treatment Procedure: Coagulation + Filter 3. Create another membrane filter (without activated charcoal). 4. Reuse the plastic bottle funnel. Fold a paper towel in half, and then fold it in half again. Separate the layers to make a funnel. Stack 3 paper napkins into a shallow bowl and tuck these into the paper funnel. Push the entire membrane construction into the funnel forming a bowl. 5. Place the coagulated wastewater in Beaker #4 into the filter. Catch the filtrate drippings in a clean beaker. Water Treatment 6. Examine the coagulation filtrate. Record the odor, color and appearance in the adjacent data table. Transfer the filtrate to the large test tube. Water Treatment 7. Measure the coagulation filtrate with the pH sensor. 8. Tap and to activate sensor to deactivate. 9. Record the result in the data table on next page. 10. Next measure the coagulation filtrate with the conductivity sensor. 12. Then measure the coagulation filtrate with the turbidity sensor. 11. Record the result in the data table on next page. 13. Record result in data table on next page. 14. Rinse beakers and test tube. Water Treatment Water Treatment Procedure: Sedimentation 1. Carefully pipette a 30 mL sample from the top of the solution in Beaker #3 (sedimentation sample), being careful not to disturb the solution. 2. Place the sedimentation sample in the test tube for analysis. Note: You will not filter this sample. Instead we will examine how effective the process of sedimentation can be in cleaning water. Water Treatment 3. Examine the sedimentation sample in the test tube. Record its odor, color and appearance in the adjacent data table. Note: After you post the data for this sample, take a picture of your completed table on pg. 41 for your journal. Water Treatment 9. Measure sedimentation 7. Next measure the sample with the turbidity sedimentation sample with sensor. the conductivity sensor. 5. Tap to activate sensor and 10. Record the result in the to deactivate. 8. Record the result in the data table on next page. data table on next page. 6. Record the result in the data 4. Measure the sedimentation sample with the pH sensor. table on next page. 11. Clean all equipment per teacher's instructions. Water Treatment Water Treatment Analysis 1. Compare your earlier predictions with your data table results. Which result surprised you the most? Please explain. Water Treatment Analysis 2. What were the effects of filtering the "wastewater" using a plain membrane filter? Water Treatment Analysis 3. What were the effects of filtering the "wastewater" using an activated-charcoal filter? Water Treatment Analysis 4. What was the effect of treatment with an coagulating/agglutinating agent? What was the effect of coagulation + membrane filtration? Water Treatment Analysis 5. What was the effect of treatment with sedimentation? How might this treatment be improved? Water Treatment Analysis 6. What quality of water is measured with the conductivity sensor? Water Treatment Analysis 7. Which treatment method worked best for eliminating odors? Water Treatment Analysis 8. Which treatment method worked best for changing the color of the water (removing the cloudiness)? Which was least effective? Water Treatment Analysis 9. What was the effect of treatment on pH? Water Treatment Analysis 10. What was the effect of treatment on conductivity (dissolved salt) levels? Water Treatment Synthesis 1. What are some differences between the treatment of water to be used for human consumption compared with treatment of wastewater to be discharged into the environment? Water Treatment Synthesis 2. Suppose you had to design a water treatment system that could reliably produce large volumes of water clean enough for safe human consumption. What treatment methods would you include? Why? Water Treatment Multiple Choice 1. The main purpose of sewage treatment is to __________. a) kill pathogenic bacteria and to reduce odor b) remove biodegradable materials from the water and kill pathogenic bacteria c) kill pathogenic bacteria and remove plant nutrients d) kill pathogenic bacteria, remove biodegradable materials, and make the water safe for human use e) None of the above. Water Treatment Multiple Choice 2. The main purpose of drinking water treatment is to __________. a) kill pathogenic bacteria and to reduce odor b) remove biodegradable materials from the water and to kill pathogenic bacteria c) kill pathogenic bacteria and remove plant nutrients d) kill pathogenic bacteria, remove biodegradable materials, and make the water safe for human use e) None of the above. Water Treatment Modern water treatment plant What happens when water treatment fails! Water Treatment Congratulations! You have completed the lab. Please remember to follow your teacher's instructions for cleaning-up and submitting your lab. Water Treatment References All images were taken from PASCO documentation, public domain clip art, or Wikimedia Foundation Commons: http://commons.wikimedia.org/wiki/File:STSTW_Effluent.jpg http://commons.wikimedia.org/wiki/File:Glass-of-water.jpg http://commons.wikimedia.org/wiki/File:Canal-pollution.jpg http://commons.wikimedia.org/wiki/File:Pfeil_rechts.svg.jpg http://commons.wikimedia.org/wiki/File:Fluor_Fernald_Workers.jpg http://commons.wikimedia.org/wiki/File:Rwenzori_mineral_water.jpg http://commons.wikimedia.org/wiki/File:Glass_of_Water.JPG http://www.freeclipartnow.com/office/paper-shredder.jpg.html