TDunlap Unit Outline and Lesson Plan
advertisement

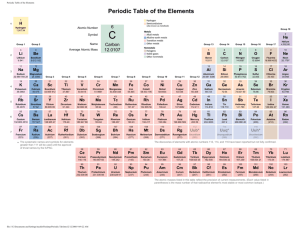
Traci Dunlap EDUC 5133 Chemistry Unit Grade Level: 8th Grade Curricular Areas: Chemistry, Mathematics, Art Time Frame: 5 weeks Goals: List and define the physical and chemical properties of matter. List and identify physical and chemical changes in matter composition. Define and visually represent an element’s atomic structure in a Bohr Model. Know the difference between protons, neutrons, and electrons. Know how elements are grouped and classified on the Periodic Table of Elements. Know how to identify elements in a substance from its chemical formula. Define and visually represent an element’s valence electrons in Lewis Dot Structure. Define and identify the different types of chemical bonds. Understand the Law of Conservation of Mass and apply its concepts to balance chemical equations. Define and identify endothermic and exothermic reactions. Objectives: Given a model of atomic structure, students will identify and define the major parts of an atom with 100% accuracy. Given a copy of the periodic table of elements, the student will translate an element’s atomic number and atomic weight into its number of protons, neutrons, and electrons with 90% accuracy. Given a group of elements on the periodic table of elements, the student will determine the physical and chemical properties of the group with 90% accuracy. Given a chemical formula, the student will correctly identify the number of elements present with 90% accuracy. Given one half of a chemical equation, the student will apply the law of conservation of mass to balance the remaining half of the equation with 90% accuracy. Given two elements, the student will determine the number of valence electrons and correctly identify the type of bond formed between the elements with 90% accuracy. Given a list of chemical equations, the student will determine if the equations are balanced or unbalanced with 100% accuracy. Given a list of elements, the student will create visual Bohr diagrams and Lewis Dot structures for each element with 100% accuracy. During an observational lab on physical and chemical properties of matter, students will complete their lab journal observations and corresponding lab report with 100% completion. During an observation lab on endothermic and exothermic reactions, students will complete their lab journal observations and corresponding lab report with 100% completion. 1 Traci Dunlap EDUC 5133 Given a series of homework assignments, students will submit all required worksheets with 100% completion. Given a series of quizzes throughout the unit, students will answer all questions with 90% accuracy. Given a unit test over all concepts discussed, students will answer all questions with 80% accuracy. Vocabulary: Atom Element Isotope Proton Neutron Electron Atomic Number Atomic Mass Bohr Model Lewis Dot Endothermic Valence Electron Valence Shell Molecule Covalent Bond Ion Ionic Bond Periodic Table Group Period Family Exothermic Physical Properties Physical Changes Chemical Properties Chemical Changes Chemical Reaction Reactant Product Compound Coefficient Subscript Law of Conservation of Mass Teaching/Learning Activities: The teacher will lead a class discussion reviewing the basic concepts of matter. The teacher will introduce the concepts of physical and chemical properties of matter complete with visual examples. The teacher will introduce the concepts of physical and chemical changes in matter composition complete with visual examples. The students will complete a lab activity where they explore physical and chemical properties of matter and observe physical changes in composition. The students will record their observations in their science journals and complete an assigned lab report on their findings. The teacher will introduce the concepts of atomic structure and Bohr models utilizing the periodic table of elements. The students will create a foldable of atomic structure. The students will complete a lab activity where they calculate the atomic structure and create a visual representation of an element. The students will complete a Periodic Table of the Elements scavenger hunt worksheet for homework. The teacher will introduce the concepts of period and group classification on the Periodic Table of the Elements and discuss the similarities of elements within the classification structure. The students will use colored pencils to color code a periodic table of elements worksheet for their science journals. 2 Traci Dunlap EDUC 5133 The students will complete worksheets identifying groups based on stated physical and chemical properties of the group members for homework. The teacher will introduce the concepts of valence electrons, Lewis Dot structure and chemical bonding. The students will draw Lewis Dot structures for a given set of elements. The students will complete a worksheet identifying the types of chemical bonds formed between atoms. The teacher will introduce the concepts of chemical formulas, subscripts and coefficients. The students will complete a lab activity creating compound models with candy and toothpicks. The students will complete a worksheet calculating the number of elements from a given list of chemical formulas for homework. The teacher will introduce the concepts of the Law of Conservation of Mass and balancing chemical equations. The students will work in groups to identify the product and reactant from a list of chemical equations and will determine if the equations are balanced or unbalanced. The students will complete a worksheet of balancing additional chemical equations for homework. The teacher will introduce the concepts of chemical reactions and how they change the properties of elements. The students will complete a lab activity focusing on endothermic and exothermic reactions and will record their observations in their science journals and complete an assigned lab report on their findings. Materials: Project Materials: o 3-D Model of Atomic Structure o Periodic Table of Elements o Bohr Model Diagrams o Science Journals o Whiteboard o Dry Erase Markers o Colored Pencils o Rubber Bands o Matches o Thermometers o Triple Beam Balance o Graduated Cylinders o Beakers o Petri Dishes o Bunsen Burners o Safety Goggles o Sodium Bicarbonate o Vinegar o Hydrochloric Acid 3 Traci Dunlap EDUC 5133 o Acetic Acid o Copper Wire o Aluminum Foil o Marbles o Rock Samples o Toothpicks o M&M Candies o Gumdrops o Magnets o Coins o Nails o Red Food Coloring o Alka Seltzer Books: o Online science textbook provided by school Electronics: o Computer o Projector o Speakers Websites: o http://www.sciencespot.net/Media/atomsfam.pdf o http://www.bozemanscience.com/atoms-the-periodic-table o http://www.bozemanscience.com/drawing-lewis-dot-diagrams o http://www.bozemanscience.com/chemical-bonds-covalent-vs-ionic o http://www.bozemanscience.com/beginners-guide-balancing-equations Evaluation/Assessments: Observation Unit Quizzes Unit Test Lab Worksheets Lab Reports Lab Models Homework Assignments Science Journal Entries Class Participation 4 Traci Dunlap EDUC 5133 Lesson Plan Title: The Atoms Family Grade Level: 8th Grade Curricular Areas: Chemistry, Mathematics, Art Time Frame: 3 class periods (45 minutes each) Materials Needed: Equipment o 3-D Model of Atomic Structure o Periodic Table of Elements o Computer o Projector o Speakers o Whiteboard o Dry Erase Markers Student material needs o Periodic Table of Elements Scavenger Hunt Worksheet o Atomic Structure Worksheet o Blank Bohr Model Worksheet o M&M candies Objectives: o Given a model of atomic structure, students will identify and define major parts of an atom with 90% accuracy. o Given a copy of the periodic table of elements, the student will translate an element’s atomic number and atomic weight into its numbers of protons, neutrons, and electrons with 90% accuracy. o Using their translation of an element’s atomic number and atomic weight, the student will create a visual diagram of an atomic model of an element complete with electron shells with 90% accuracy. o Given a model of atomic structure, students will be able to correctly identify the element depicted with 90% accuracy. o Given a quiz on atoms and atomic structure, students will answer all questions with 90% accuracy. TEKS: Science (3) Scientific Investigation and reasoning. The student uses critical thinking, scientific reasoning, and problem solving to make informed decision and knows the contributions of relevant scientists. The student is expected to: o (B) use models to represent aspects of the natural world such as an atom, a molecule, space or a geologic feature; 5 Traci Dunlap EDUC 5133 (5) Matter and energy. The student knows that matter is composed of atoms and has chemical and physical properties. The student is expected to: o (A) describe the structure of atoms, including the masses, electrical charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud; Mathematics (14) Underlying processes and mathematical tools. The student applies Grade 8 mathematics to solve problems connected to everyday experiences, investigations in other disciplines, and activities in and outside of school. The student is expected to: o (A) identify and apply mathematics to everyday experiences, to activities in and outside of school, with other disciplines, and with other mathematical topics; Art Perception. The student develops and organizes ideas from the environment. The student is expected to: o (A) illustrate ideas from direct observation, imagination, and personal experience and from experiences at school and community events; Context/Modifications Prior Knowledge: o Students should be able to define matter and identify the three main states of matter (solid, liquid, gas). o Students should have a basic knowledge of laboratory rules and safety procedures. Modifications for Students with Special Needs o Priority seating for students with visual/hearing impairments or other learning disabilities who require close proximity to the teacher to follow the lesson. o Provide an outline/study guide of the lesson prior to class for the special needs learner to review. o Allow tape recording of the lecture. o Simplify the worksheets and/or allow the use of calculators for solving math calculations. o Provide one-on-one assistance during group work to facilitate greater understanding of the topic. o Read individual IEP’s to identify specific assistive technology or accommodations required for student participation. o Coordinate with special education personnel for additional accommodations as needed. Modifications for English Language Learners o Provide a glossary of terms used in the lecture. o Provide a worksheet for note taking. o Closely observe ELL during the lesson to ensure they understand the lecture and activity. o Pair the ELL with an English speaking partner to help facilitate comprehension of the lecture and activity. 6 Traci Dunlap EDUC 5133 o Use a combination of visual/auditory elements to teach the lesson. o Coordinate with an ESL specialist for additional accommodations as needed. o Allow the ESL specialist to directly assist student during the class period. Anticipatory Focusing: The teacher will deliver a PowerPoint presentation on The Atoms Family which will tell a story of Protons, Neutrons and Electrons. The presentation will include a song to be sung by the students that will incorporate basic concepts of atomic structure. Setting Expectations: Following the PowerPoint presentation, the teacher will inform the students that for the next three days they will be studying atoms and atomic structure. The teacher will review the TEKs and objectives for the lesson with the students and will highlight relevant terms listed on their unit vocabulary summary created at the beginning of the unit. The teacher will provide students with a table of contents for the lesson to be included in their science journals. Each required artifact will the entered into the table of contents at the start of the lesson. The teacher will stress that all homework assignments and lab activities will need to be completed and present in the science journals before being able to take the end of unit exam. The teacher will inform the students they will take a quiz within four days focusing on concepts learned during the lesson. The teacher will review stated lab rules and safety requirements for the students to observe during the lab activity. Input: PowerPoint presentation on atomic structure (The Atoms Family) YouTube video on Bohr Model Class discussions Chapter reading from textbook Unit vocabulary summary created at the beginning of the unit Modeling: Following the YouTube video on the Bohr Model, the teacher will model a calculation of atomic structure for the class. Prior to the lab activity, the teacher will construct a Bohr Model diagram with M&M candies to model the activity for the students. 7 Traci Dunlap EDUC 5133 Checking for Understanding: Following the PowerPoint presentation, students will be shown a 3-D model of an atom and asked to identify its parts. The students will assemble and label an atomic structure foldable for their science journal. The foldable will be assessed for accuracy by the teacher prior to completion as homework. Following the YouTube video on the Bohr Model, students will participate in a classroom discussion where they will be questioned on the information presented in the video. Guided Practice: For a pre-lab activity, the students will be given worksheets depicting various elements from the Periodic Table. They will be asked to calculate the atomic structure: number of protons, neutrons and electrons from the atomic number and mass provided on the table. The teacher will evaluate each student’s responses for accuracy before proceeding to the lab activity. The students will complete a lab activity creating a Bohr Model of atomic structure with M&M candies. The teacher will evaluate each group’s responses for accuracy before completing the lab activity. Reteach: If students are unable to correctly identify the parts of the atom on the 3-D model, the teacher can review the Atoms Family presentation before retesting the students’ knowledge. If students continue to show confusion with their calculations during the pre-lab activity, the teacher can model one to two additional calculations for the class before reassessing the group worksheets. Independent Practice: The student will complete a Periodic Table of Elements scavenger hunt worksheet for homework. The student will create an atomic structure foldable for their science journal during class and will complete it for homework. Mastery Check: The students will need to complete their pre-lab worksheets with 90% accuracy before progressing to the Bohr Model lab activity. The teacher will check all homework assignments for completion and accuracy. The class will take a unit quiz within two class periods of completing the activity. The unit quiz will define the parts of the atom, calculate atomic structure, diagram and label the atomic structure of an element, and identify an element from a given diagram. The unit quiz will formally assess mastery of the concepts practiced during the lesson. 8 Traci Dunlap EDUC 5133 Extension: Required for G/T students, optional for rest: o Students will research and complete a worksheet on isotopes. o Students will research and complete a worksheet on electron orbitals. Closure: Following the Bohr Model lab activity, the students will participate in a classroom discussion reviewing all the concepts taught during the lesson in preparation for a unit quiz. Appropriate materials to be included in the science journal will be reviewed for completion and accuracy. The students will take a unit quiz assessing mastery of the lesson material. Reflective Critique: Was there enough time to present the lesson at a pace comfortable for all students? Did the students require any math remediation to perform the required calculations? Did the students seemed overwhelmed with any of the information presented during lecture? Did the students enjoy The Atoms Family presentation? Did the students understand the Bohr Model presentation? Did the students actively participate and show comprehension of the material during class discussions? Did the students complete homework assignments accurately? Did the students’ quiz scores reflect the required mastery of the material? How often did the students need to have information repeated or clarified? Was there enough time during the lesson for the students to ask questions and have information clarified if needed? Did the students observe required laboratory rules and safety procedures? Integration of Technology: Use of computer with accompanying A/V input PowerPoint Presentation o http://www.sciencespot.net/Media/atomsfam.pdf YouTube Video on Bohr Model o http://www.bozemanscience.com/atoms-the-periodic-table 9