IB Biology Handbook 2015-16
advertisement

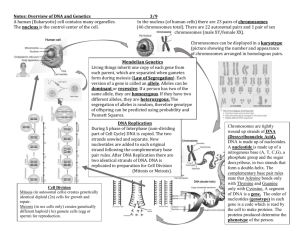
Nicholas Senn High School IB Biology DP Handbook 1 Table of Contents: Topic IB Learner Profile IB Biology Course of Study IB Biology Assessments Sample mark bands Rules for drawing on the Exam Internal Assessment: Grade criteria and Overview IA: Lab report guidelines IA: Doing statistics in Excel and on Graphing calculators Safety guidelines in the lab Measuring protocols IB Action Verbs Summary of Topics and Timelines Syllabus and page numbers Page 3 4 5 6-7 8 9-12 13-17 17-20 21-22 23-25 26 27-28 29-36 2 IB Learner Profile Inquirers Knowledgeable Thinkers Communicators Principled Open-minded Caring Risk-takers Balanced Reflective They develop their natural curiosity. They acquire the skills necessary to conduct inquiry and research and show independence in learning. They actively enjoy learning and this love of learning will be sustained throughout their lives. They explore concepts, ideas and issues that have local and global significance. In so doing, they acquire in-depth knowledge and develop understanding across a broad and balanced range of disciplines. They exercise initiative in applying thinking skills critically and creatively to recognize and approach complex problems, and make reasoned, ethical decisions. They understand and express ideas and information confidently and creatively in more than one language and in a variety of modes of communication. They work effectively and willingly in collaboration with others. They act with integrity and honesty, with a strong sense of fairness, justice and respect for the dignity of the individual, groups and communities. They take responsibility for their own actions and the consequences that accompany them. They understand and appreciate their own cultures and personal histories, and are open to the perspectives, values and traditions of other individuals and communities. They are accustomed to seeking and evaluating a range of points of view, and are willing to grow from the experience. They show empathy, compassion and respect towards the needs and feelings of others. They have a personal commitment to service, and act to make a positive difference to the lives of others and to the environment. They approach unfamiliar situations and uncertainty with courage and forethought, and have the independence of spirit to explore new roles, ideas and strategies. They are brave and articulate in defending their beliefs. They understand the importance of intellectual, physical and emotional balance to achieve personal well-being for themselves and others. They give thoughtful consideration to their own learning and experience. They are able to assess and understand their strengths and limitations in order to support their learning and personal development. 3 IB Biology Course of Study 4 Assessment in IB Biology There are two main types of assessment in IB: internal and external. Internal assessment (IA) is work that is graded by your Senn High School Biology teacher. The external assessments are the tests you take at the end of your senior year. How well you do on both the internal and external assessments will determine whether or not you receive the IB diploma. Standard Level (SL) Component Overall weight (%) Paper 1 20 Duration (hours) ¾ Format and Syllabus Coverage 30 multiple-choice questions on the Core (Chapters 1-6) Section A: One data-based question and several short-answer questions on the Core (Chapters 1-6). All questions must be completed. Paper 2 Paper 3 IA 40 20 20 Higher Level (HL) Component Overall weight (%) 1¼ 1 60 Duration (hours) Paper 1 20 1 Paper 2 36 2¼ Paper 3 IA 24 20 1¼ 60 Section B: One extended response questions on the Core (Chapters 1-6). You choose one question (Part A, B, and C) from a choice of two. Section A: Two to three short-answer questions based on experimental skills and techniques, analysis and evaluation, using data linked to the core material. Section B: Short-answer and extended-response questions from one option. The highest scoring lab from all independent labs completed will be used for the IA grade. Format and Syllabus Coverage 40 multiple-choice questions on the Core plus additional higher level (AHL) (Chapters 1-11) Section A: One data-based question and several short-answer questions on the Core plus AHL (Chapters 1-11). All questions must be completed. Section B: Two extended response questions on the Core plus AHL (Chapters 1-11). You choose two question (Part A, B, and C) from a choice of three. Section A: Two to three short-answer questions based on experimental skills and techniques, analysis and evaluation, using data linked to the core material. Section B: Short-answer and extended-response questions from one option. The highest scoring lab from all independent labs completed will be used for the IA grade. 5 Sample Mark Bands for each of the Assessment sections SL/HL 6 7 Rules for Drawing on an Exam: 1. 2. 3. 4. 5. 6. 7. Draw in pencil Make the drawing at least 1/3 page large Label with straight lines with a ruler Lines must exactly touch the structure which is named. If there is a doubt, no points will be awarded Include a title Size of parts must be correct in relation to the larger drawing Print all labels horizontally on the drawing Example of a cell drawing given full marks: 8 Common Vocabulary Variables o Independent variable: what you change (as an independent person) – it influences results o Dependent variable: what you measure – it depends on the independent variable o Controlled variables: the factors that must be kept the same in order to do a “fair test” Set-up o The way equipment and materials are used o Includes equipment, which should usually be shown with a diagram o Includes the amounts of materials; unless the amount of a material is the independent variable, the amounts will stay the same from one trial to the next . Trials o Exact repetitions of a set up o Data may be single results (1 reading per trial) or it may be a set of results as when a number of readings are taken over a given period of time Control (the control) o A set up in which the independent variable is absent o A set of data that should be the norm. What you can use for comparison with your experimental data Experimental versus Control set-ups o In the experiment a hypothesis is tested by manipulating the independent variable. o In the control, the independent variable is not manipulated. This is done to eliminate the possibility that an effect might result from a source other than the independent variable. Continuous/ Discontinuous data o This may not be as much a matter of using the right vocabulary as it is establishing the idea that some data points can logically be connected with the assumption that intermediate data could have been taken. o This is a graphing concern o Expamples: climatograms, which are graphs with 2 sets of data on one graph: temperature and precipitation. Temperature from month to month is continuous. I.e., if the average temperature for May is 15 degrees and the average temperature for June is 18 degrees, somewhere in between the temperatures between 15 and 18 were represented. Precipitation, however, is discontinuous, since it is given as the total for each month, and each month has a distinct number. Raw/ Processed data o Raw data is the actual data you collect during the lab. You have not done any mathematical processes to the data. It should be listed with units and significant figures. o Processed data is data after it has been manipulated mathematically. Raw data should be processed before it is graphed (or otherwise presented). For instance, means are considered rather than all data points. 9 IB SCORING CRITERIA FOR IA (2014 onward) 10 11 12 IB Lab Guidelines Part 1: Background Aim (research question)—make it clear what you are investigating. This should include why and how. Include what you think will happen and why. Include what you already know about the topic. If you need to site your sources. Include the independent variable, dependent variables, and controlled variables (one of which should be temperature). When listing variables be specific. For the variables (IV and DV) include the units they will be measured in (if appropriate) and then the range of values you will use (for IV). The controlled variables should include units and possible effects where applicable and a sentence as to how each variable will be controlled. Make a table like this in your lab background section: Part II: Procedure (Method) Includes method for collecting data, which includes details of how to measure your independent and dependent variables Each step should be specified so that another person could follow the procedure. Correct measuring procedures should be included. Give details of values, units and equipment Make sure you describe collecting enough data (at least five trials for each set-up) Make sure you have a sufficient range (for example if your IV is temperature that you are testing a wide enough range of temperatures). Take a digital photo or draw your set-up and label all of the equipment Include how the method for controlling the control variables (example: temperature can be controlled with a water bath). 13 Solute concentration (%) (+/- 0.5%) 0 3 6 Trial Part III. Recording raw data (Observations) Record in a data table that has labels, title, and units Include qualitative (what you see/hear/feel/smell) and quantitative data (numbers with units) Include possible errors and uncertainties in your measurement Uncertainties are +/- ½ of the smallest increment the measuring device can measure if it is nonelectronic. If it is electronic it is +/- the smallest increment. Weight of disc before (g) (+/- 0.5 g) Weight after (g) (+/- 0.5 g) Weight change % change (g) (+/- 1%) Mean % change (+/- 1%) Observations 1 2 3 4 5 1 2 3 4 5 1 2 3 4 5 Part IV: Processing data/processed data (Evidence) Most common is averaging (mean). The most difficult part of this section is to choose the appropriate type of processing for your data Statistical tests are often used such as SD, and t-test o SD show the spread of the data around the mean SD should be used if you have at least 5 repeats of an experimental group SD should be drawn as error bars on your graph If the error bars overlap the experimental groups are not that different from each other o T-test shows if two groups of data are significantly different from each other. Need a 95% confidence level Can only do if have two experimental groups Include an example of any calculations performed. Put the processed data into a graph and table—make sure they are labeled with units/uncertainties labels and titles Graphs need to include error bars X-axis is independent variable. Y-axis is dependent variable. Title is usually “The effect of _____________ (IV) on ___________________ (DV)” Types of graphs: Bar graphs: if your IV is categories not numbers with units Scatter graphs: If your IV is numbers with units (continuous variable) Pie graphs: If you data is % out of a whole (all the numbers add up to 100%) 14 Part V: Conclusion Paragraph 1: What you found out Claim—what is your answer to your question? Evidence—How does your data support your claim? (What general trends do you observe? What do they suggest?) In this part you need to specifically reference your data. You cannot reference observations or data that you do not have explicitly stated in your data section. Is the claim and evidence similar to what you though would happen? Refer to your background. Are there any unusual results (outliers)? What might be their significance? Why might they have been caused? If you have a reason from your experimental observations then they need to be referenced. Do other sources of information support your findings? This would be reasoning for why the evidence supports your claim. How does this data relate to the broader body of science research? (Cite your source) 15 For Paragraph 1: Some terms to discuss your data: Ways to cite data (use evidence to support a claim): Paragraph 2: Evaluating your procedure (Sources of error) What difficulties did you encounter with the procedure when conducting the lab? What were some inaccuracies that could have occurred when conducting the lab? What did you do to minimize adverse effects on data collection? Did anything occur during the investigation to compromise your data? Types of Error: Human Error: tools or instruments are read incorrectly (let the thermometer touch the container, did not read at eye level..). All instruments should be read at eye level. These can be because the experimenter does not know how to use the instruments or random because the experimenter lost concentration during the experiment. Using probes that collect data for you can reduce these errors. Instrument Error: The equipment/measuring device might not be accurate. This can be due to lack of calibration or lack of measuring precision. The equipment should be checked for accuracy, this includes calibration of all instruments. For example, calibrating balances, using a blank in the colorimeter, calibrating DO probes. Also attempt to use equipment that can measure more precisely. For example a ruler that can measure mm instead of one that can only measure .5 cm. Random Errors: There is variation in all biological phenomena. For example the concentration of solutes within a potato might vary based on part of a potato or which potato is used. To reduce this type of error a couple things can be done: use a control set-up to compare your data to, control all other variables (i.e. use the same potato, use water baths to control temp), and increasing the number of replicates (at least 5). Procedural Error: When a measurement is taken, this can affect the environment of the experiment. For example, when a cold thermometer is put in a test tube of warm water, the water will be cooled by the presence 16 of the thermometer, or when the behavior of animals is being recorded the present of the experimenter may influence the animals’ behavior. Paragraph 3: Improving the investigation For each weakness or limitation listed describe a workable method to fix the problem What are extensions you could do to further your investigation? Using Excel: General Rules: If you number does not right justify (shift automatically to the right of the cell then the computer did not recognize it as a number If you put any letters into cells the computer will not recognize them as numbers—DO NOT PUT UNITS IN CELLS Don’t use Excel to make data tables—use word Put similar data into columns Put X values in the first column, Y values in the next column. Use the toolbar to select additional changes to data Use a scatter plot not a line graph Simple Statistics: (when it says range—put the cell number and letter separated by a colon) =Average (range) =Median (range) =Mode (range) =STDEV (range) 17 Graphing Data 1. Enter the data into columns 2. Select the data 3. Click on Chart from the menu bar 4. In Graph Type select the type you want. Choose ‘Column’ for bar charts or ‘XY scatter’ for line and scatter graphs. Do not choose ‘line’. 5. If you graph looks wrong you can correct it by clicking on the series tab and selecting the data you want (X and Y values) 6. In Chart Options select the choice that allows you to label the axes and have a title. You might also want the option that puts in a line of best fit. 7. You can also turn on and off gridlines and legends. 8. You can also add error bars by selecting “Format Data Series.” You want to choose custom error bars. Under both the + and – select the STDEV for your data series. Sometimes error bars can be used to show what type of trend line better fits the data. 9. You can add trend lines by double clicking on any data point. Then select to add a trend line. You can decide what type of trend line you would like to add. You can also choose to add it in the Chart Options (step 6). The formula and correlation coefficient can also be added to the chart. 18 T-test: Used to compare two sets of normally-distributed data. This determines if the sets are significantly different. =TTest (range 1, range 2, tails, type) This gives the P-value directly Tails: (either a value of 1 or 2) In biology we generally want the two-tailed t-test, which tests for differences regardless of sign. Type: (either a value of 1 or 2). If the data is paired, meaning it is from the same individuals then the number is 1. If the data is unpaired; the sets are from two individuals, then the number is 2. 19 Using the TI-84 for Statistics The calculator can be used to enter data from experiments. This data can then be manipulated to produce statistics such as mean and standard deviation as well as chi-square and student T-tests. All of these statistical topics are included in the syllabus as indicated by the brackets. Basic Statistics: Mean & Standard Deviation Data is stored in lists. If data is in the lists from previous experiments the lists will need to be cleared. If no data is present, you are ready to begin the problem at Part A. Press the STAT button. Either press 4; OR use the cursor keys to move down to ClrList and press enter You will now need to specify which list you want to clear. Use the blue or yellow 2nd button to choose your list. Press 2nd and then press 1. Press ENTER. You will now have cleared list 1 and you are free to enter the data. a) Press the STAT button again. The Edit function is highlighted by default. Press ENTER. Now enter the following data by pressing enter after each number. 9 enter 7 enter 6 enter 10, 4, 5, 6, 7, 9, 6, 9, 8, 7, 8, 5 b) Press the STAT button again. Use the blue cursor arrows so that CALC is highlighted. Press ENTER to calculate a number of single variable statistics. You must specify which list you want to do the calculation for, so press 2nd then press the 1 to indicate that you want the statistics for L1 and then press ENTER. You should see that the mean is given as 7.1, the standard deviation is 1.75, the number of statistics is 15 etc. Student’s t-test (Topic 1) Example from Allott Biology for the IB Diploma: To test whether there was any difference in the size of lichens growing on the top and side of a stone wall, some data were collected. The diameters of a random sample of ten lichens on the top and ten on the side were measured and a t-test was used to find out if there was a significant difference. Surface Top Side Diameter of lichen (mm) 22 10 24 45 9 26 5 34 10 13 22 12 23 13 7 13 5 24 3 10 a. b. c. d. e. f. Clear your L1 as specified in the instructions above. Clear your L2 using the same procedure. Enter the Top data into L1 and the Side data into L2. Push the STAT button Move the cursor to the TESTS menu. Press 4; the default should be Data (it will be flashing), Pooled should be highlighted as Yes (Pooled data means the data came from one source) g. Move the cursor down to the last row off the screen until you see the flashing Calculate. Press ENTER. Despite the means being 19.8 and 13.2 respectively, a t value of 1.41 with a (p) value of 17.7% shows that the difference in means is not statistically significant. 20 Safety Guidelines What are the safety Do’s and Don’ts for Students? Life threatening injuries can happen in the laboratory. For that reason, students need to be informed of the correct way to act and things to do in the laboratory. The following is a safety checklist. Conduct: Do not engage in practical jokes or boisterous conduct in the laboratory Never run in the laboratory The performance of unauthorized experiments is strictly forbidden Do not sit while performing experiments General Work Procedure Never work in the laboratory without the supervision of a teacher Immediately report any spills, accidents, or injuries to a teacher Never leave experiments while in progress Never attempt to catch a falling object Be careful when handling hot glassware and apparatus in the laboratory. Hot glassware looks just like cold glassware. Never fill a pipette using mouth suction. Always use a pipetting device Make sure no flammable solvents are in the surrounding area when lighting a flame Do not leave a lit Bunsen burner unattended Turn off all heating apparatus, gas valves, and water faucets when not in use. Do not remove any equipment or chemicals form the laboratory. Coats, bags and other personal items must be stored in designated areas, no on the bench tops or in the aisle ways. Notify your teacher of any sensitivities that you may have to particular chemicals if known. Housekeeping: Keep work area neat and free of any unnecessary objects Thoroughly clean your laboratory work space at the end of the laboratory session Do not block the sink drains with debris Never block access to exits or emergency equipment Inspect all equipment for damage (cracks, defects..) prior to use; do not use damaged equipment Place chemical waste in appropriately labeled waste containers Properly dispose of broken glassware and other sharp objects immediately in designated containers Properly dispose of weigh boats, gloves, filter paper, and paper towels in the laboratory Apparel in the Lab Always wear goggles Wear disposable gloves, as provided in the lab, when handling hazardous materials. Remove gloves before exiting the lab Wear shoes that adequately cover the whole foot; low-heeled shoes with non-slip soles are preferable. Do not wear sandals, open-toed shoes, open-backed shoes, or high-heeled shoes in the lab Secure long hair and loose clothing Remove jewelry (especially dangling jewelry) Synthetic finger nails are not recommended in the lab; they are made of extremely flammable polymers 21 Hygiene Practices Keep your hands away from your face, eyes, mouth and body while using chemicals Food and drink, open or closed, should never be brought into the lab or chemical storage area Never use lab glassware for eating or drinking purposes Do not apply cosmetics while in the lab Wash hands after removing gloves and before leaving the lab Remove any protective equipment before leaving the lab Chemical Handling Check the label to verify it is the correct substance before using it Wear appropriate chemical resistant gloves before handling chemicals If you transfer chemicals form their original containers, label chemical containers as to the contents, concentration, hazard, date and your initials Always use a spatula or scoopula to remove a solid reagent from a container Do not directly touch any chemical with your hands Hold containers away from the body when transferring a chemical or solution from one container to another Use a hot water bath to heat flammable liquids. Never heat directly with a flame Add concentrated acid to water slowly. Never add water to a concentrated acid. Weigh out or remove only the amount of chemical you will need. Do not return the excess to its original container, but properly dispose of it in the appropriate waste container Never touch, taste or smell any reagents Never place the container directly under your nose and inhale the vapors Never mix or use chemicals not called for in the lab exercise Clean up all spills properly and promptly as instructed by the teacher Dispose of chemicals as instructed by the teacher 22 Measuring Protocols/Laboratory Techniques Pouring liquids 1. Use the back of your fingers to remove the stopper from a reagent bottle. Hold the stopper between your fingers until the transfer of liquid is complete. Do not place the stopper on your workbench. 2. Grasp the container from which you are pouring with the palm of your hand covering the label. 3a. When you are transferring a liquid to a test tube or measuring cylinder, the container should be held at eye level. Pour the liquid slowly, until the correct volume has been transferred. 3b. When you are pouring a liquid from a reagent bottle into a beaker, the reagent should be poured slowly down a glass stirring rod. When you are transferring a liquid from one beaker to another, you can hold the stirring rod and beaker in one hand. Filtering a Mixture Sometimes it is necessary to separate a solid from a liquid. The most common method of separating such a mixture is filtration. 1. Fold a filter paper circle in half and then quarters. Open the folded paper to form a cone, with one thickness of paper on one side and three thicknesses on the other. 2. Put the paper cone in a filter funnel. Place the funnel in an iron ring clamped to a ring stand. Moisten the filter paper with a small volume of distilled water, and gently press the paper against the sides of the funnel to achieve a good fit. (If the correct size of filter paper has been used, the top edge of the cone will be just below the rim of the filter funnel.) 3. Place a beaker beneath the funnel to collect the filtrate. The tip of the funnel should touch the inside surface of the beaker and extend about one inch below the rim. Guide flow of liquid with a glass rod Mixture being filtered Filtrate Solid collects on filter paper Stem touches side of beaker. 4. Decant the liquid from the solid by pouring it down a glass stirring rod into the funnel. Be careful to keep the liquid below the top edge of the cone of filter paper at all times; the liquid must not overflow. Finally, use a jet of distilled water from a wash bottle to wash the solid into the filter. 5. When the filtration is complete, wash the solid residue on the filter paper with distilled water to remove traces of solvent. Dry the solid. 6. If the filtrate contains a dissolved salt, it may be recovered by evaporation if desired. Using a Gas Burner Laboratory gas burners produce various kinds of flames when different mixtures of gas and air are burned. The two most common models are the Bunsen burner and the Tirrell burner. Both have adjustable air vents; the Tirrell burner has a gas control valve in its base. 1. Examine your laboratory burner. Determine which model you have. 2. Connect the burner to the gas supply with rubber tubing. 3. Close the air vents. If your model is a Tirrell burner, also close the gas control valve at the base of the burner. 4. Hold a lighted match at the top of the burner tube and turn on the gas supply. Do this by opening the main gas supply valve located on top of the nozzle to which you attached the rubber tubing. (If your model is a Tirrell burner, first open the main gas supply valve, then open the gas control valve at the base approximately onehalfturn.) You should get a yellow, or luminous, flame. When a Tirrell burner is used, the main gas supply valve should be opened fully and the gas flow regulated by the gas control valve. Gas supply to a Bunsen burner is controlled by the main gas valve. 5. Open the air vents slowly, to admit more air into the flame, to produce a light blue (nonluminous) coneshaped flame. If the flame “blows out” after lighting, the gas supply should be reduced. 6. Adjust the air vents and gas supply to produce the desired size of flame. For most laboratory work, the blue inner cone of the flame should be about 1 inch high and free of yellow color. If you want a smaller flame, close the air vent slightly and reduce the gas supply. You will learn how to control the burner flame by trial and error. 7. Turn the burner off at the main gas supply valve when done. CAUTION: Confine long hair and loose 23 clothing when using a gas burner. Do not reach over a burner. Ensure that flammables are not being used when a burner is lit. Never leave a lit burner unattended. Know the location of fire extinguishers, the fire blanket, and safety shower. Heating Liquids Heating a Liquid in a Test Tube The correct procedure for heating liquids in the laboratory is important to laboratory safety. 1. Adjust your gas burner to produce a gentle blue flame. 2. Fill a test tube one-third full with the liquid to be heated. 3. Grasp the test tube with a test-tube holder, near the upper end of the tube. 4. Hold the test tube in a slanting position in the flame, and gently heat the tube a short distance below the surface of the liquid. 5. Shake the tube gently as it is being heated, until the liquid boils or reaches the desired temperature. CAUTION: Never point the open end of a test tube you are heating either toward yourself or anyone working nearby. Never heat the bottom of the test tube. Heating a Liquid in a Beaker Many laboratory experiments require the use of a hot water or boiling water bath. This procedure describes how to assemble a water bath. Instead of a gas burner you can use a large beaker on a hot plate. 1. Fasten an iron ring securely to a ring stand so that it is 2–4 cm above the top of a gas burner placed on the ring stand base. 2. Place a 250-mL beaker one-half-filled with water on a wire gauze resting on the iron ring. 3. Light your gas burner and adjust it to produce a hot flame. 4. Place the burner beneath the wire gauze. For a slower rate of heating, reduce the intensity of the burner flame. CAUTION: Never heat plastic beakers or graduated glassware in a burner flame. Never let a boiling water bath boil dry; add water to it as necessary. Measuring Mass In many experiments you are required to determine the mass of a chemical used or produced in a reaction. An object’s mass is determined by measuring it on a balance. When you determine the mass of an object, you are comparing its mass with a known mass. In the SI, the base unit of mass is the kilogram. 1. Check the balance before you start. The balance pan should be empty and clean, and all masses (or dials) should be set on zero. The balance must be level. Check the bubble level on the base. See your teacher if you need assistance with checking your balance. 2. Objects to be placed directly on the balance pan must be clean, dry, and at room temperature. Solid chemicals and liquids must never be put directly on the balance pan. Liquid samples should be placed in beakers or sealed containers. Solid chemicals can be conveniently placed in beakers, disposable plastic weighing boats, or on 10cm squares made of glossy paper. 3. The balance is a precision instrument that must be handled with care. To avoid damaging it, always be sure that the balance is in an arrested position when objects are placed on or removed from the pan. 4. Never move or jar either a balance or the balance table. 5. If you spill a chemical on or near the balance, clean it up immediately. If in doubt, inform your teacher. A camel-hair brush is usually provided to wipe minute traces of solid from the balance pan before you use it. 6. Never attempt to measure an object with a mass greater than the maximum capacity of the balance. 7. When you are done, return all the masses to zero, and make sure the balance pan is clean. Do not attempt to use a balance until your teacher has demonstrated the proper technique. 24 Measuring Volume Volume measurements are important in many experimental procedures. Sometimes volume measurements must be accurate; other times they can be approximate. Most volume measures in the laboratory are made using equipment calibrated in milliliters. Although some beakers have graduation marks, these marks are designed only for quick, rough estimates of volume. Accurate volumes must be measured with pipets, burets, or volumetric flasks. Using a Graduated Cylinder Half-fill a 100-mL graduated cylinder with water, and set the cylinder on your laboratory bench. Examine the surface of the water. Notice how the surface curves upward where the water contacts the cylinder walls. This curved surface is called a meniscus. A volume measurement is always read at the bottom of the meniscus, with your eye at the same level as the liquid surface. To make the meniscus more visible, you can place your finger or a dark piece of paper behind and just below the meniscus while making the reading. Graduated cylinders are available in many capacities. The 100-mL cylinder is marked in 1-mL divisions, and volumes can be estimated to the nearest 0.1 mL. The last digit in these measurements is therefore significant but uncertain. Using a Pipet A pipet is used to accurately measure and deliver volumes of liquids. Two types are in common use: volumetric pipets and graduated, or measuring, pipets. The use of a volumetric pipet will be described. A volumetric pipet has a single calibration mark and delivers the volume printed on the bulb of the pipet at the temperature specified. (A graduated pipet has calibrations along the length of the pipet.) Volumes can be measured more accurately with a volumetric pipet than with a graduated pipet. 1. Place the tip of the pipet below the surface of the liquid to be dispensed. 2. Compress a pipet bulb and press the hole in the bulb against the upper end of the pipet. CAUTION: Never fill a pipet by applying suction with your mouth. Never push the pipet bulb over the end of the pipet. 3. Slowly release pressure on the bulb so that liquid is drawn into the pipet to a level about 2 cm above the calibration mark. 4. Remove the bulb and simultaneously place your index finger over the end of the pipet. If you are righthanded, you should hold the pipet in your right hand and the pipet bulb in your left. 5. Keep your index finger pressed firmly against the end. Withdraw the pipet from the liquid, and carefully wipe the outside of the stem with a paper towel. 6. Slowly reduce the pressure on your finger to allow the excess liquid to drain into a waste receiver, until the bottom of the meniscus is at the calibration mark. 7. Now, deliver the remaining liquid in the pipet into the designated receiver. When releasing liquid from a volumetric pipet, let it drain completely. Wait 20 seconds, then touch the pipet tip to the side of the flask or surface of the liquid. This action will remove some, but not all, of the liquid in the tip. The pipet delivers the stated volume when this procedure is followed. A small amount of liquid remains in the tip. Do not blow this out into your receiver. Using a Thermometer Thermometers need to be read at eye level. The bottom of the thermometer needs to be in the liquid only it should not touch the bottom or sides of a container. It must always be read in Celsius. 25 IB Action Verbs Objective 1: Define: Give the precise meaning of a word or phrase as concisely as possible Draw: represent by means of pencil lines (add labels unless told not to do so) at least 1/3 of a page List: give a sequence of names or other brief answers with no elaboration, each one clearly separated from the others Measure: find a value for a quantity State: give a specific name, value or other brief answer (no supporting argument or calculation is necessary) Objective 2: Annotate: add brief notes to a diagram, drawing or graph Apply: use an idea, equation, principle, theory or law in a new situation Calculate: find an answer using mathematical methods (show the working unless instructed not to do so) Compare: give an account of similarities and differences between two (or more) items, referring to both (all) of them throughout (comparisons can be given using a table) Describe: give a detailed account, including all the relevant information Distinguish: give the differences between two or more different items Estimate: find an appropriate value for an unknown quantity, based on the information provided and scientific knowledge Identify: find an answer from a number of possibilities Outline: give a brief account or summary (include essential information only) Objective 3: Analyze: interpret data to reach conclusions Construct: represent or develop in graphical form Deduce: manipulate a mathematical equation to give a new equation or result Design: produce a plan, object, simulation or model Determine: find the only possible answer Discuss: give an account including, where possible, a range of arguments, assessments of the relative importance of various factors or comparisons of alternative hypotheses Evaluate: assess the implications and limitations Explain: give a clear account including causes, reasons or mechanisms Predict: give an expected result Solve: obtain an answer using algebraic and/or numerical methods Suggest: propose hypotheses or other possible answer. 26 Summary of Topics and Timeline: Diploma Program Course Core topics are required for all IB biology students (standard and higher level). Two option topics are also required for all students. Additional higher level (AHL) is required only for higher level students. Semester 1 Content (Grade 11) Chapter Topics -- Statistical Analysis (Core) 4 Ecology (Core) (communities, ecosystems, green house effect, populations, carbon cycle) 5 Evolution and Biodiversity (Core) (natural selection, cladistics, classification) 1 Cell Biology (Core) (cell theory, cell structure, origin of cells) 2 Molecular Biology (Core) (chemical elements and water, carbohydrates, lipids, and proteins, enzymes) 7 Nucleic Acids and Proteins (AHL) (proteins and enzymes) Semester 2 Content (Grade 11) Chapter 1 Topics Cell Biology (core) (Membranes, cell division) 2 Molecular Biology (Core) (photosynthesis and cell respiration, DNA structure, replication, transcription and translation) **IA Lab ** 7 Nucleic Acids (AHL) (DNA structure, replication, transcription, translation) 3 Genetics (Core) (chromosomes, mutations, meiosis, theoretical genetics, genetic engineering and biotechnology) 10 Genetics and Evolution (AHL) (meiosis, inheritance, dihybrid cross, gene linkage, polygenic inheritance, gene pools and speciation) 27 Semester 1 Content (Grade 12) – Subject to Change for 2015-2016 course Chapter 6 A Topics Human Physiology (Core) (digestion, transport system, defense against infectious disease, gas exchange, nerves, hormones, homeostasis, reproduction) **Group IV Project** Neurobiology and Behavior (Core/AHL) (Neural development, the human brain, perception of stimuli, innate and learned behavior, neuropharmacology, ethology) Semester 2 Content (Grade 12) Chapter 11 9 8 Topics Animal Physiology (AHL) (immunity, muscles, kidney, reproduction) Plant Biology (AHL) (structure, growth, transport, reproduction) Metabolism, Cell Respiration, and Photosynthesis (AHL) (chemical reactions in detail) 28 Syllabus Assessment Statements and Page Numbers (Year 1 ONLY) The revised IB Biology course syllabus for exams beginning in 2016 divides course content into three major categories: Understandings, Applications, and Skills: Understandings list the general life sciences content to be covered in the course. Applications identify specific scientific cases and examples to which understandings should be applied. Skills describe investigations, activities, and techniques that students should be able to do. Section Understandings / Applications / Skills 1.1: Introduction to Cells (Page 1) 1.1.12 1.1.13 1.1.14 According to the cell theory, living organisms are composed of cells Organisms consisting of only one cell carry out all functions of life in that cell Surface are to volume ratio is important in the limitation of cell size Multicellular organisms have properties that emerge from the interaction of their cellular components Specialized tissues can develop by cell differentiation in multicellular organisms Differentiation involves the expression of some genes and not others in the cell’s genome The capacity of stem cells to divide and differentiate along different pathways is necessary in embryonic development. It also makes stem cells suitable for therapeutic purposes. Question the cell theory using atypical examples, including striated muscle, giant algae and aseptate fungal hyphae. Investigate functions of life in Paramecium and one named photosynthetic unicellular organism. Describe the use of stem cells to treat Stargardt’s disease and one other named condition. Discuss the ethics of therapeutic use of stem cells from specially-created embryos, from the umbilical cord blood of a new-born baby, and from an adult’s own tissue. Use a light microscope to investigate the structure of cells and tissues. Draw cell structures as seen with the light microscope. Calculate the magnification of drawings and the actual size of structures shown in drawings or micrographs. 1.2.1 1.2.2 1.2.3 1.2.4 1.2.5 1.2.6 1.2.7 1.2.8 1.2.9 Prokaryotes have a simple cell structure without compartments Eukaryotes have a compartmentalized cell structure. Prokaryotes divide by binary fission. Electron microscopes have a much higher resolution than light microscopes. The structure and function of organelles within the exocrine gland cells of the pancreas. The structure and function of organelles within palisade mesophyll cells of the leaf. Draw the ultrastructure of prokaryotic cells based on electron micrographs. Draw the ultrastructure of eukaryotic cells based on electron micrographs. Interpret electron micrographs to identify organelles and deduce the function of specialized cells. 1.3.1 1.3.2 1.3.3 1.3.4 1.3.5 1.3.6 1.3.7 Phospholipids form bilayers in water due to the amphipathic properties of phospholipid molecules. Membrane proteins are diverse in terms of structure, position in the membrane and function. Cholesterol is a component of animal cell membranes. Cholesterol in mammalian membranes reduces membrane fluidity and permeability to some solutes. Draw the fluid mosaic membrane model Analysis of evidence from electron microscopy that led to the proposal of the Davson-Danielli model. Analysis of the falsification of the Davison-Danielli model that led to the Singer-Nicolson model. 1.4.1 1.4.2 1.4.3 1.4.4 Particles move across membranes by simple diffusion, facilitated diffusion, osmosis, and active transport. The fluidity of membranes allows materials to be taken into cells by endocytosis or released by exocytosis. Vesicles move materials within cells. Describe the structure and function of sodium-potassium pumps for active transport and potassium channels for facilitated diffusion in axons. 1.1.1 1.1.2 1.1.3 1.1.4 1.1.5 1.1.6 1.1.7 1.1.8 1.1.9 1.1.10 1.1.11 1.2: Ultrastructure of Cells (Page 16) 1.3: Membrane Structure (Page 25) 1.4: Membrane Transport (Page 33) 29 1.4.5 1.4.6 Understand that tissues or organs to be used in medical procedures must be bathed in a solution with the same osmolarity as the cytoplasm to prevent osmosis. Estimate osmolarity in tissues by bathing samples in hypotonic and hypertonic solutions. 1.5: The Origins of Cells (Page 45) 1.5.1 1.5.2 1.5.3 1.5.4 Cells can only be formed by division of pre-existing cells. The first cells must have arisen from non-living material. The origin of eukaryotic cells can be explained by the endosymbiotic theory. Evidence from Pasteur’s experiments that spontaneous generation of cells and organisms does not now occur on Earth. 1.6.1 1.6.2 1.6.3 1.6.4 1.6.5 1.6.6 1.6.7 1.6.8 1.6.9 Mitosis is division of the nucleus into two genetically identical daughter nuclei. Chromosomes condense by supercoiling during mitosis. Cytokinesis occurs after mitosis and is different in plant and animal cells. Interphase is a very active phase of the cell cycle with many processes occurring in the nucleus and cytoplasm. Cyclins are involved in the control of the cell cycle. Mutagens, oncogenes, and metastasis are involved in the development of primary and secondary tumors. Identify the correlation between smoking and incidence of cancers. Identify phases of mitosis in cells viewed with a microscope. Determine a mitotic index from a micrograph. 2.1.1 2.1.2 2.1.3 2.1.4 2.1.5 Molecular biology explains living processes in terms of the chemical substances involved. Carbon atoms can form four bonds allowing a diversity of compounds to exist. Life is based on carbon compounds including carbohydrates, lipids, proteins, and nucleic acids. Metabolism is the web of all the enzyme catalyzed reactions in a cell or organism. Anabolism is the synthesis of complex molecules from simpler molecules including the formation of macromolecules from monomers by condensation reactions. Catabolism is the breakdown of complex molecules into simpler molecules including the hydrolysis of macromolecules into monomers. Urea as an example of a compound that is produced by living organisms but can also be artificially synthesized. Draw molecular diagrams of glucose, ribose, a saturated fatty acid, and a generalized amino acid. Identify biochemicals as carbohydrates, lipids, or proteins from molecular diagrams. 1.6: Cell Division (Page 51) 2.1: Molecules to Metabolism (Page 61) 2.1.6 2.1.7 2.1.8 2.1.9 2.2: Water (Page 68) 2.2.1 2.2.2 2.2.3 2.2.4 2.2.5 2.2.6 Water molecules are polar and hydrogen bonds form between them. Hydrogen bonding and dipolarity explain the adhesive, cohesive, thermal, and solvent properties of water. Substances can be hydrophilic or hydrophobic. Compare the thermal properties of water with those of methane. Explain the use of water as a coolant in sweat. Methods of transport of glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride in blood in relation to their solubility in water. 2.3.1 2.3.2 2.3.3 2.3.4 2.3.5 2.3.6 2.3.7 2.3.8 2.3.9 2.3.10 Monosaccharide monomers are linked together by condensation reactions to form disaccharides and polysaccharide polymers. Fatty acids can be saturated, monounsaturated, or polyunsaturated. Unsaturated fatty acids can be cis or trans isomers. Triglycerides are formed by condensation from three fatty acids and one glycerol. Structure and function of cellulose and starch in plants and glycogen in humans. Scientific evidence for health risks of trans-fats and saturated fats. Lipids are more suitable for long-term energy storage in humans than carbohydrates. Evaluation of evidence and the methods used to obtain evidence for health claims made about lipids. Use of molecular visualization software to compare cellulose, starch, and glycogen. Determination of body mass index by calculation or use of a nomogram. 2.4.1 2.4.2 Amino acids are linked together by condensation to form polypeptides. There are twenty different amino acids in polypeptides synthesized on ribosomes. 2.3: Carbohydrates and Lipids (Page 73) 2.4: Proteins (Page 87) 30 2.4.3 2.4.4 2.4.5 2.4.6 2.4.7 2.4.8 2.4.9 2.4.10 2.4.11 Amino acids can be linked together in any sequence giving a huge range of possible polypeptides. The amino acid sequence of polypeptides is coded for by genes. A protein may consist of a single polypeptide or more than one polypeptide linked together. The amino acid sequence determines the three dimensional conformation of a protein. Living organisms synthesize many different proteins with a wide range of functions. Every individual has a unique proteome. Identify rubisco, insulin, immunoglobulins, rhodopsin, collagen, and spider silk as examples of the range of protein functions. Explain the denaturation of proteins by heat or deviation of pH from the optimum. Draw molecular diagrams showing the formation of a peptide bond. 2.5: Enzymes (Page 96) 2.5.1 2.5.2 2.5.3 2.5.4 2.5.5 2.5.6 2.5.7 Enzymes have an active site to which specific substrates bind. Enzyme catalysis involves molecular motion and the collision of substrates with the active site. Temperature, pH, and substrate concentration affect the rate of activity of enzymes. Enzymes can be denatured. Immobilized enzymes are widely used in industry. Methods of production of lactose-free milk and its advantages. Design of experiments to test the effect of temperature, pH, and substrate concentration on the activity of enzymes. 2.6.1 2.6.2 The nucleic acids DNA and RNA are polymers of nucleotides. DNA differs from RNA in the number of strands normally present, the base composition, and the type of pentose. DNA is a double helix made of two antiparallel strands of nucleotides linked by hydrogen bonding between complimentary base pairs. Crick and Watson’s elucidation of the structure of DNA using model-making. Draw simple diagrams of the structure of single nucleotides and of DNA and RNA, using circles, pentagons, and rectangles to represent phosphates, pentoses, and bases. 2.6: Structure of DNA & RNA (Page 105) 2.6.3 2.6.4 2.6.5 2.7: DNA Replication, Transcription, and Translation (Page 111) 2.7.1 2.7.2 2.7.3 2.7.4 2.7.5 2.7.6 2.7.7 2.7.8 2.7.9 2.7.10 2.7.11 2.7.12 2.7.13 2.7.14 The replication of DNA is semi-conservative and depends on complimentary base-pairing. Helicase unwinds the double helix and separates the two strands by breaking hydrogen bonds. DNA polymerase links nucleotides together to form a new strand, using the pre-existing strand as a template. Transcription is the synthesis of mRNA copied from the DNA base sequence by RNA polymerase. Translation is synthesis of polypeptides on ribosomes. The amino acid sequence of polypeptides is determined by mRNA according to the genetic code. Codons of three bases on mRNA correspond to one amino acid in a polypeptide. Translation depends on complimentary base pairing between codons of mRNA and anticodons on tRNA. Use of Taq DNA polymerase to produce multiple copies of DNA rapidly by the polymerase chain reaction (PCR). Production of human insulin in bacteria as an example of the universality of the genetic code allowing gene transfer between species. Use a table of the genetic code to deduce which codon(s) correspond to which amino acid. Analysis of Meselson and Stahl’s results to obtain support for the theory of semi-conservative replication of DNA. Use a table of mRNA codons and their corresponding amino acids to deduce the sequence of amino acids coded by a short mRNA strand of known base sequence. Deducing the DNA base sequence for the mRNA strand. 2.8: Cell Respiration (Page 122) 2.8.1 2.8.2 2.8.3 2.8.4 2.8.5 2.8.6 2.8.7 Cell respiration is the controlled release of energy from organic compounds to produce ATP. ATP from cell respiration is immediately available as a source of energy in the cell. Anaerobic cell respiration gives a small yield of ATP from glucose. Aerobic cell respiration requires oxygen and gives a large yield of ATP from glucose. Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking. Lactate production in humans when anaerobic respiration is used to maximize the power of muscle contractions. Analysis of results from experiments involving measurement of respiration rates in germinating seeds or invertebrates using a respirometer. 31 2.9: Photosynthesis (Page 129) 2.9.1 2.9.2 2.9.3 2.9.4 2.9.5 2.9.6 2.9.7 2.9.8 2.9.9 2.9.10 Photosynthesis is the production of carbon compounds in cells using light energy. Visible light has a range of wavelengths with violet the shortest wavelength and red the longest. Chlorophyll absorbs red and blue light most effectively and reflects green light more than other colors. Oxygen is produced in photosynthesis from photolysis of water. Energy is needed to produce carbohydrates and other carbon compounds from carbon dioxide. Temperature, light intensity, and carbon dioxide concentration are possible limiting factors on the rate of photosynthesis. Changes to the Earth’s atmosphere, oceans, and rock deposition due to photosynthesis. Design experiments to investigate limiting factors on photosynthesis. Separation of photosynthetic pigments by chromatography. Draw an absorption spectrum for chlorophyll and an action spectrum for photosynthesis. 3.1: Genes (Page 141) 3.1.1 3.1.2 3.1.3 3.1.4 3.1.5 3.1.6 3.1.7 3.1.8 3.1.9 3.1.10 A gene is a heritable factor that consists of a length of DNA and influences a specific characteristic. A gene occupies a specific position (locus) on one type of chromosome. The various specific forms of a gene are alleles. Alleles differ from each other by one or a few bases only. New alleles are formed by mutation. The genome is the whole of the genetic information of an organism. The entire base sequence of human genes was sequenced in the Human Genome Project. The causes of sickle cell anemia, including a base substitution mutation, a change to the base sequence of mRNA transcribed from it, and a change to the sequence of a polypeptide in hemoglobin. Comparison of the number of genes in humans with other species. Use of a database to determine differences in the base sequence of a gene in two species. 3.2: Chromosomes (Page 149) 3.2.1 3.2.2 3.2.3 3.2.4 3.2.5 3.2.6 3.2.7 3.2.8 3.2.9 3.2.10 3.2.11 3.2.12 3.2.13 3.2.14 3.2.15 Prokaryotes have one chromosome consisting of a circular DNA molecule. Some prokaryotes also have plasmids but eukaryotes do not. Eukaryotic chromosomes are linear DNA molecules associated with histone proteins. In a eukaryotic species there are different chromosomes that carry different genes. Homologous chromosomes carry the same sequence of genes but not necessarily the same alleles of those genes. Diploid nuclei have pairs of homologous chromosomes. Haploid nuclei have one chromosome of each pair. The number of chromosomes is a characteristic feature of members of a species. A karyogram (karyotype) shows the chromosomes of an organism in homologous pairs of decreasing length. Sex is determined by sex chromosomes and autosomes are chromosomes that do not determine sex. Cairn’s technique for measuring the length of DNA molecules by autoradiography. Comparison of genome size in T2 phage, Escherichia coli, Drosophila melanogaster, Homo sapiens, and Paris japonica. Comparison of diploid chromosome numbers of Homo sapiens, Pan troglodytes, Canis familiaris, Oryza sativa, and Parascaris equorum. Use of karyotypes to deduce sex and diagnose Down syndrome in humans. Use of online databases to identify locus of a human gene and its protein product. 3.3: Meiosis (Page 159) 3.3.1 3.3.2 3.3.3 3.3.4 3.3.5 3.3.6 3.3.7 3.3.8 3.3.9 3.3.10 One diploid nucleus divides by meiosis to produce four haploid nuclei. The halving of the chromosome number allows a sexual life cycle with fusion of gametes. DNA is replicated before meiosis so that all chromosomes consist of two sister chromatids. The early stages of meiosis involve pairing of homologous chromosomes and crossing over followed by condensation. Orientation of pairs of homologous chromosomes prior to separation is random. Separation of pairs of homologous chromosomes in the first division of meiosis halves the chromosome number. Crossing over and random orientation promotes genetic variation. Fusion of gametes from different parents promotes genetic variation. Non-disjunction can cause Down syndrome and other chromosomal abnormalities. Studies showing age of parents influences chances of non-disjunction. Methods used to obtain cells for karyotype analysis (chorionic villus sampling and amniocentesis) and the 32 3.3.11 associated risks. Draw diagrams to show the stages of meiosis resulting in the formation of four haploid cells. 3.4: Inheritance (Page 168) 3.4.15 3.4.16 3.4.17 Mendel discovered the principles of inheritance with experiments in which large numbers of pea plants were crossed. Gametes are haploid so contain one allele of each gene. The two alleles of each gene separate into different haploid daughter nuclei during meiosis. Fusion of gametes results in diploid zygotes with two alleles of each gene that may be the same allele or different alleles. Dominant alleles mask the effects of recessive alleles but co-dominant alleles have joint effects. Many genetic diseases in humans are due to recessive alleles of autosomal genes. Some genetic diseases are sex-linked and some are due to dominant or co-dominant alleles. The pattern of inheritance is different with sex-linked genes due to their location on sex chromosomes. Many genetic diseases have been identified in humans but most are very rare. Radiation and mutagenic chemicals increase the mutation rate and can cause genetic disease and cancer. Inheritance of ABO blood groups. Red-green color-blindness and hemophilia as examples of sex-linked inheritance. Inheritance of cystic fibrosis and Huntington’s disease. Consequence of radiation after nuclear bombing of Hiroshima and Nagasaki and the nuclear accident at Chernobyl. Construct Punnett grids (squares) for predicting the outcomes of monohybrid genetic crosses. Comparison of predicted and actual outcomes of genetic crosses using real data. Analysis of pedigree charts to deduce the pattern of inheritance of genetic diseases. 3.5.1 3.5.2 3.5.3 3.5.4 3.5.5 3.5.6 3.5.7 3.5.8 3.5.9 3.5.10 3.5.11 3.5.12 3.5.13 3.5.14 3.5.15 Gel electrophoresis is used to separate proteins or fragments of DNA according to size and charge. PCR can be used to amplify small amounts of DNA. DNA profiling involves comparison of DNA. Genetic modification is carried out by gene transfer between species. Clones are groups of genetically identical organisms, derived from a single original parent cell. Many plant species and some animal species have natural methods of cloning. Animals can be cloned at the embryo stage by breaking up the embryo into more than one group of cells. Methods have been developed for cloning adult animals using differentiated cells. Use of DNA profiling in paternity and forensic investigations. Gene transfer to bacteria with plasmids using restriction endonucleases (enzymes) and DNA ligase. Assessment of the potential risks and benefits associated with genetic modification of crops. Production of cloned embryos by somatic cell nuclear transfer. Design an experiment to assess one factor affecting the rooting of stem cuttings. Analysis of examples of DNA profiles. Analysis of data on risks to monarch butterflies of Bt crops. 4.1.1 4.1.2 4.1.3 4.1.4 4.1.5 4.1.6 4.1.7 4.1.8 4.1.9 4.1.10 4.1.11 4.1.12 Species are groups of organisms that can potentially interbreed to produce fertile offspring. Members of a species may be reproductively isolated in separate populations. Species have either an autotrophic or heterotrophic method of nutrition (a few species have both). Consumers are heterotrophs that feed on living organisms by ingestion. Detritivores are heterotrophs that obtain organic nutrients from detritus by internal digestion. Saprotrophs are heterotrophs that obtain organic nutrients from dead organic matter by external digestion. A community is formed by populations of different species living together and interacting with each other. A community forms an ecosystem by its interactions with the abiotic environment. Autotrophs and heterotrophs obtain inorganic nutrients from the abiotic environment. The supply of inorganic nutrients is maintained by nutrient cycling. Ecosystems have the potential to be sustainable over long periods of time. Classify species as autotrophs, consumers, detritivores, or saprotrophs from a knowledge of their mode of nutrition. Test for association between two species using a chi-squared test with data obtained from quadrat sampling. Recognize and interpret statistical significance. Setting up sealed mesocosms to try to establish sustainability. 3.4.1 3.4.2 3.4.3 3.4.4 3.4.5 3.4.6 3.4.7 3.4.8 3.4.9 3.4.10 3.4.11 3.4.12 3.4.13 3.4.14 3.5: Genetic Modification & Biotechnology (Page 187) 4.1: Species, Communities, and Ecosystems (Page 201) 4.1.13 4.1.14 4.1.15 33 4.2: Energy Flow (Page 213) 4.2.1 4.2.2 4.2.3 4.2.4 4.2.5 4.2.6 4.2.7 4.2.8 Most ecosystems rely on a supply of energy from sunlight. Light energy is converted to chemical energy in carbon compounds by photosynthesis. Chemical energy in carbon compounds flows through food chains by means of feeding. Energy released by respiration is used in living organisms and converted to heat. Living organisms cannot convert heat to other forms of energy. Heat is lost from ecosystems. Energy losses between trophic levels restrict the length of food chains and the biomass of higher trophic levels. Quantitative representations of energy flow using pyramids of energy. 4.3.1 4.3.2 4.3.3 4.3.4 4.3.5 Autotrophs convert carbon dioxide into carbohydrates and other carbon compounds. In aquatic habitats carbon dioxide is present as a dissolved gas and hydrogen carbonate ions. Carbon dioxide diffuses from the atmosphere or water into autotrophs. Carbon dioxide is produced by respiration and diffuses out of organisms into water or the atmosphere. Methane is produced from organic matter in anaerobic conditions by methanogenic archaeans and some diffuses into the atmosphere. Methane is oxidized to carbon dioxide and water in the atmosphere. Peat forms when organic matter is not fully decomposed because of anaerobic conditions in waterlogged soils. Partially decomposed organic matter from past geological eras was converted into oil and gas in porous rocks or into coal. Carbon dioxide is produced by the combustion of biomass and fossilized organic matter. Animals such as reef-building corals and mollusks have hard parts that are composed of calcium carbonate and can become fossilized in limestone. Estimate carbon fluxes due to processes in the carbon cycle. Analysis of data from atmosphere monitoring stations showing annual fluctuations. Construct a diagram of the carbon cycle. 4.3: Carbon Cycling (Page 220) 4.3.6 4.3.7 4.3.8 4.3.9 4.3.10 4.3.11 4.3.12 4.3.13 4.4: Climate Change (Page 229) 4.4.1 4.4.2 4.4.3 4.4.4 4.4.5 4.4.6 4.4.7 4.4.8 4.4.9 4.4.10 4.4.11 Carbon dioxide and water vapor are the most significant greenhouse gases. Other gases, including methane and nitrogen oxides have less impact as greenhouse gases. The impact of a gas depends on its ability to absorb long-wave radiation as well as on its concentration in the atmosphere. The warmed Earth emits longer-wave radiation (heat). Longer-wave radiation is reabsorbed by greenhouse gases which retains the heat in the atmosphere. Global temperatures and climate patterns are influenced by concentrations of greenhouse gases. There is a correlation between rising atmospheric concentrations of carbon dioxide since the start of the industrial revolution two hundred years ago and average global temperatures. Recent increases in atmospheric carbon dioxide are largely due to increases in the combustion of fossilized organic matter. Correlations between global temperatures and carbon dioxide concentrations on Earth. Evaluate claims that human activities are not causing climate change. Threats to coral reefs from increasing concentrations of dissolved carbon dioxide. 5.1: Evidence for Evolution (Page 241) 5.1.1 5.1.2 5.1.3 5.1.4 5.1.5 5.1.6 5.1.7 5.1.8 Evolution occurs when heritable characteristics of a species change. The fossil record provides evidence for evolution. Selective breeding and domesticated animals shows that artificial selection can cause evolution. Evolution of homologous structures by adaptive radiation explains similarities in structure when there are differences in function. Populations of a species can gradually diverge into separate species by evolution. Continuous variation across the geographical range of related populations matches the concept of gradual divergence. Compare the pentadactly limb of mammals, birds, amphibians, and reptiles with different methods of locomotion. Development of melanistic insects in polluted areas. 5.2: Natural Selection (Page 249) 5.2.1 Natural selection can only occur if there is variation amongst members of the same species. 34 5.2.2 5.2.3 5.2.4 5.2.5 5.2.6 5.2.7 5.2.8 5.2.9 Mutation, meiosis, and sexual reproduction cause variation between individuals in a species. Adaptations are characteristics that make an individual suited to its environment and way of life. Species tend to produce more offspring than the environment can support (overproduction). Individuals that are better adapted tend to survive and produce more offspring while the less well adapted tend to die or produce fewer offspring. Individuals that reproduce pass on characteristics to their offspring. Natural selection increases the frequency of characteristics that make individuals better adapted and decreases the frequency of other characteristics leading to changes within the species. Describe changes in beaks of finches on Daphne Major. Describe evolution of antibiotic resistance in bacteria. 5.3: Classification & Biodiversity (Page 258) 5.3.1 5.3.2 5.3.3 5.3.4 5.3.5 5.3.6 5.3.7 5.3.8 5.3.9 5.3.10 5.3.11 5.3.12 5.3.13 The binomial system of names for species is universal among biologists and has been agreed and developed at a series of congresses. When species are discovered they are given scientific names using the binomial system. Taxonomists classify species using a hierarchy of taxa. All organisms are classified into three domains. The principal taxa for classifying eukaryotes are kingdom, phylum, class, order, family, genus, and species. In natural classification the genus and accompanying higher taxa consist of all the species that have evolved from one common ancestral species. Taxonomists sometimes reclassify groups of species when new evidence shows that a previous taxon contains species that have evolved from different ancestral species. Classifications help in identification of species and allow the prediction of characteristics shared by species within a group. Classification of one plant and one animal species from domain to species level. External recognition features of bryophytes, filicinophytes, coniferophytes, and angiospermophytes. Recognition of features of porifera, cnidaria, platyhelmenthes, annelida, mollusca, arthropoda, and chordata. Recognition of features of birds, mammals, amphibians, reptiles, and fish. Construct dichotomous keys for use in identifying specimens. 5.4: Cladistics (Page 263) 5.4.1 5.4.2 5.4.3 5.4.4 5.4.5 5.4.6 5.4.7 5.4.8 5.4.9 A clade is a group of organisms that have evolved from a common ancestor. Evidence for which species are part of a clade can be obtained from the base sequences of a gene or the corresponding amino acid sequence of a protein. Sequence differences accumulate gradually so there is a positive correlation between the number of differences between two species and the time since they diverged from a common ancestor. Traits can be analogous or homologous. Cladograms are tree diagrams that show the most probable sequence of divergence in clades. Evidence from cladistics has shown that classification of some groups based on structure did not correspond with the evolutionary origins of a group or species. Analyze cladograms including humans and other primates. Discuss reclassification of the figwort family using evidence from cladistics. Analyze cladograms to deduce evolutionary relationships. 7.1: DNA Structure & Replication AHL (Page 343) 7.1.1 7.1.2 7.1.3 7.1.4 7.1.5 7.1.6 7.1.7 7.1.8 7.1.9 7.1.10 7.1.11 DNA structure suggested a mechanism for DNA replication. Nucleosomes help to supercoil the DNA. DNA replication is continuous on the leading strand and discontinuous on the lagging strand. DNA replication is carried out by a complex series of enzymes. DNA polymerases can only add nucleotides to the 3’ end of a primer. Some regions of DNA do not code for proteins but have other important functions. Discuss Rosalind Franklin’s and Maurice Wilkins’ investigation of DNA structure by X-ray diffraction. Explain how tandem repeats are used in DNA profiling. Use of nucleotides containing dideoxyribonucleic acid to stop DNA replication in preparation of samples for base sequencing. Analysis of results of the Hershey and Chase experiment providing evidence that DNA is the genetic material. Utilize molecular visualization software to analyze the association between protein and DNA within a nucleosome. 35 7.2: Transcription & Gene Expression AHL (Page 355) 7.2.1 7.2.2 7.2.3 7.2.4 7.2.5 7.2.6 7.2.7 7.2.8 Gene expression is regulated by proteins that bind to specific base sequences in DNA. The environment of a cell and of an organism has an impact on gene expression. Nucleosomes help to regulate transcription in eukaryotes. Transcription occurs in a 5’ to 3’ direction. Eukaryotic cells modify mRNA after transcription. Splicing of mRNA increases the number of different proteins an organism can produce. Describe the promoter as an example of non-coding DNA with a function. Analyze changes in DNA methylation patterns. 7.3.1 7.3.2 7.3.3 7.3.4 7.3.5 7.3.6 7.3.7 7.3.8 7.3.9 Initiation of translation involves assembly of the components that carry out the process. Synthesis of the polypeptide involves a repeated cycle of events. Disassembly of the components follows termination of translation. Free ribosomes synthesize proteins for use primarily within the cell. Bound ribosomes synthesize proteins primarily for secretion or for use in lysosomes. Translation can occur immediately after transcription in prokaryotes due to the absence of a nuclear membrane. The sequence and number of amino acids in the polypeptide is the primary structure. The secondary structure is the formation of alpha helices and beta pleated sheets stabilized by hydrogen bonds. The tertiary structure is the further folding of the polypeptide stabilized by interactions between R groups (sidechains). The quaternary structure exists in proteins with more than one polypeptide chain. tRNA-activating enzymes illustrate enzyme-substrate specificity and the role of phosphorylation. Use molecular visualization software to analyze the structure of eukaryotic ribosomes and a tRNA molecule. Identify polysomes in an electron micrograph. 7.3: Translation AHL (Page 362) 7.3.10 7.3.11 7.3.12 7.3.13 10.1 Meiosis AHL (page 439) 10.1.1 10.1.2 10.1.3 10.1.4 10.1.5 10.1.6 10.1.7 10.1.8 Chromosomes replication in interphase before meiosis Crossing over is the exchange of DNA material between non-sister homologous chromatids Chiasmata formation between non-sister chromatids in a bivalent can result in an exchange of alleles. Crossing over produces new combinations of alleles on the chromosome of the haploid cells. Homologous chromosomes separate in meiosis Independent assortment of genes is due to the random orientation of pairs of homologous chromosomes in meiosis I Sister chromatids separate in meiosis II Drawing diagrams to show chiasmata formed by crossing over. 10.2 Inheritance AHL (page 445) 10.2.10 10.2.11 Unlinked genes segregate independently as a result of meiosis Gene loci are said to be linked if on the same chromosome Variation can be discrete or continuous The phenotypes of polygenic characteristics tend to show continuous variation Chi-squared tests are used to determine whether the difference between an observed and expected frequency distribution is statistically significant Completion and analysis of Punnett squares for dihybrid traits Morgan’s discovery of non-Mendelian ratios in Drosophila Polygenic traits such as human height may also be influenced by environmental factors Calculations of the predicted genotypic and phenotypic ratio of offspring of dihybrid crosses involving unlinked autosomal genes Identification of recombinants in crosses involving two linked genes Use of a chi-squared test on data from dihybrid crosses 10.3.1 10.3.2 10.3.3 10.3.4 10.3.5 A gene pool consists of all the genes and their different alleles, present in an interbreeding population Evolution requires that allele frequencies change with time in populations Reproductive isolation of populations can be temporal, behavioral or geographic Speciation due to divergence of isolated populations can be gradual Speciation can occur abruptly 10.2.1 10.2.2 10.2.3 10.2.4 10.2.5 10.2.6 10.2.7 10.2.8 10.2.9 10.3 Gene pools and speciation AHL (page 455) 36 10.3.6 10.3.7 10.3.8 Identifying examples of directional, stabilizing and disruptive Speciation in the genus Allium by polyploidy Comparison of allele frequencies of geographically isolated populations Sources: http://www.saburchill.com/IBbiology/sci_invest/007.html British school IB Biology handbook CDC chemical safety guidelines for high schools http://www.cdc.gov/niosh/docs/2007-107/pdfs/2007-107.pdf Statistics for Biology in Excel Laboratory Technique http://www.fairbornchempage.com/Resources/Lab%20safety%20packet.htm 37