Heating Land Water Lab college style
advertisement

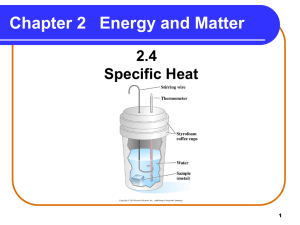
AIM: How does water and sand (land) compare in their ability to heat up and cool down? OBJ: Given college style lab SWBAT explain how water and sand (land) compare in their ability to heat up and cool down with 70% accuracy. Heating and Cooling of Land & Water Lab DN: Introduction to lab; expectation ACT: 1] collect temperature and time data 2] graph results (double line graph) 3] write-up college style lab report (including data table, graph, abstract form) 4] answer all questions. HW: Complete HCLW lab write-up (due Tuesday); Work on Meteorology Activity Sheet, Meteorology Exam, Oct 28, Interim, Nov. 6 (10%). IMPORTANT SAFETY: Heat lamps are HOT!! Do not touch lamp directly. The heating of land and water is related to the specific heat of the substance. We will investigate this concept by going to http://dictionary.reference.com/ using our iPad. In the search bar, type in: specific heat. Specific Heat: the number of calories required to raise the temperature of 1 gram of a substance 1°C. Specific Heat of Substances http://www.engineeringtoolbox.com/specific-heat-capacity-d_391.html Specific Heat of Water: Specific Heat of Sand (dry): 4182 J/kg·ºc 830 J/kg·ºc COLLEGE STYLE LAB REPORT • Problem: How does water and sand (land) compare in their ability to heat up and cool down? Hypothesis: educated guess • Materials: equipment used • Procedure: Steps taken to solve problem. • Results/Conclusion: What did you learn/discover? See Temperature vs. Time double line graph • Abstract/Summary: Briefly explain . . . – Why was lab done? – Briefly state steps/experiments done. – What did you find/learn/discover? (Hint: see data table, graph; be specific). Materials: ??? Procedure: Steps taken. How did you set up the experiment? Comments: Given the materials above, construct an experiment to: 1] test the rate at which water heat’s up and cool down, and 2] test the rate at which soil heat’s up and cools down. You are given a blank data table to collect your data and blank graph paper to plot your data. The graph will be a double line graph (1st line = water; 2nd line = soil) with one axis temperature and the other axis time. You are given 20 minutes to collect your heating and cooling data for soil (land) and water. You will be given 5 minutes to discuss and set up your soil versus water experiment. We will start at Time = 0, record the temperature. Then I will start the stopwatch on the smartboard and every minute I will announce the time. You will record your temperature (ºC) & time data. At 20 minutes, the experiment is over. Plot your results and start the lab write up. READY? http://www.online-stopwatch.com/full-screen-stopwatch/ DATA TABLE GRAPHING RULES - For each axis: equally spaced intervals - For each axis: continuous numbering ex: 1, 2, 3, 4, 5 4, 8, 12, 16, 20 2, 4, 6, 8, 10 5, 10, 15, 20, 25 3, 6, 9, 12, 15 6, 12, 18, 24, 30 - Label axes variable and units ex: Time (minutes), Distance (meter) - Connect data points making a line - Title for graph Constant – equipment that remains the same during an experiment. Control - a set-up in an experiment in which the factor shows no change; used for comparison Dependent/ Responding Variable you can not control; measure; data/information collected; y-axis Independent/Manipulating Variable you control; the factor that is changing (being tested); x-axis Results/Conclusion 1. What is the total change in temperature for water? ____ ºC For soil? ____ ºC 2. Which substance had the greater change in temperature? _________ 3. Which substance heated up faster? Why? 4. Which substance heated up slower? Why? 5. Based on your results, which do you think will heat up more quickly on a sunny day: The water in the lake or the sand surrounding it? After dark, which will cool off more quickly? SUMMARY • • • • • • Land (sand): heats up and cools _______ Water (ocean): heats up and cools ______ Dark: Light: Smooth surfaces: Rough surfaces: ABSTRACT FORM State three science concepts that you learned based on this lab: WRONG STATEMENTS – Does not tell teacher what you have learned. 1. I learned about mass and how to calculate mass. 2. I learned about volume and how to find volume. 3. I learned about density and how to calculate density. CORRECT STATEMENTS: 1. I learned that mass is the amount of matter (atoms) in a substance and it is measured using a balance (unit: grams). 2. I learned that volume is the space an object occupies and for cubic/ rectangular solids we use a ruler where V = L x W x H; for irregular objects (cork, pebble) we use a graduated cylinder (water displacement method) to find volume. 3. I learned that density is the amount of mass divided by the amount of volume; it represents the internal atomic arrangement of atoms in a substance.