Your Report
advertisement

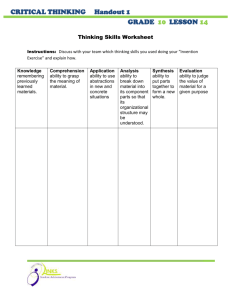
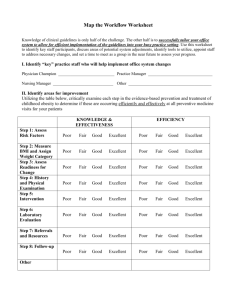
Lab #24 Write-Up (Due 5-29-13) Rubric Your Report Header (REDOX Reaction) Objective Procedure & Materials: Refer to Handout Data: Observations and Drawing on Lab Data sheet. Discussion: Conclusion Answer questions on worksheet. Errors/Improvements Claim – given on handout; Evidence – refer to observations and drawing; Science Concept – define ox/red and solutions chemistry. Lab Worksheet Lab #23 Write-Up (Due 5-20-13) Rubric Your Report Header (Effect Conc. on Rxn Rate) Objective Procedure & Materials: Refer to Handout Data: Tables 1 & 2 (in report) and Graphs 1 & 2 Calculations: Dilution Equation; Instantaneous Rate (Graph 1) Discussion: Conclusion Errors/Improvements and question Claim – given on handout; Evidence (Refer to graph) Science Concept (rate & conc (collision theory); reaction mechanisms; indicators forming a colored complex; activation complex, etc.) Lab Worksheet Lab #22 Write-Up (Due 5-10-13) Rubric Your Report Header (Establishing Equilibrium) Objective Procedure & Materials: Refer to Handout Data: Graph attached Discussion: Conclusion Observations # 1-4 (in report) ; Analysis #1-5 (on worksheet) Errors/Improvements Claim – given on handout; Evidence (refer to graph; rate as slope) Science Concept (dynamic equilibrium; rate; concentration; etc.) Lab Worksheet Lab #21 Write-Up (Due 4-22-13) Rubric Your Report Header (Titration of NaOH) Objective Procedure & Materials: Refer to Handout Data: Refer to Tables in Handout Calculations: Show equation (on WS), substitution w/units, answer w/units for Discussion: all titration calculations. Label average value as final answer. Conclusion Questions (Neatly answer on back of handout.) Errors/Improvements (Write in report.) Claim – given on handout; Evidence - refer to data and calculations. Science Concept – include questions listed in the handout. Lab Handout & Data Sheet Lab #20 Activity Rubric Lab Worksheet (Due 4-15-13) Electrolytes Complete Dissociation Equations Answer Questions (check) Lab#19 Write-Up (Due 4-12-13) Rubric Your Report Header (Making a Solution) Objective Procedure & Materials: Refer to Handout Data: Table attached Discussion: Conclusion Errors/Improvements (on WS) (on WS) Claim – given on handout; Evidence (signs of rxn-refer to table) Science Concept (activity table, ionic compounds (aq); signs of rxn) Lab Worksheet Lab #18 Activity Rubric Lab Worksheet (Due 4-15-13) Electrolytes Complete Dissociation Equations Answer Questions (check) Lab #17 Write-Up Rubric Your Report Header (Double Replacement Reactions) Objective Procedure & Materials: Refer to Handout Data: Refer to Handout for 1.) Observation Table, 2.) Table of Formula Discussion: Equations predicting products, and 3.) Four handwritten Ionic Equations (full and net; identify spectator ions by crossing out) Errors/Improvements Conclusion (Due 3-19-13) (Paragraph form) Claim – given on handout; Evidence (observations to formula products to ionic equations; evidences) Science Concept (solubility -Table F, precipitate, ionic compounds and aqueous; double replacement rxn.) Lab Worksheet Lab #15 Activity Rubric Lab Worksheet (Due 3-6-13) Conservation During a Chemical Reaction Answer Questions Lab#15 Write-Up (Due 3-7-13) Rubric Your Report Header (Single Replacement Reactions) Objective Procedure & Materials: Refer to Handout Data: Table attached Discussion: Conclusion Errors/Improvements Claim – given on handout; Evidence (signs of rxn-refer to table) Science Concept (activity table, ionic compounds (aq); signs of rxn) Lab Worksheet Lab #14 Write-Up (Due 2-26-13) Rubric Your Report Header (3-D Molecular Models) Objective: Write in report Procedure & Materials: Refer to Handout Data: Table attached Discussion: Conclusion Questions #1-6; Errors/Improvements Claim – given on handout; Evidence (Tables- explain VSEPR and shape) Science Concept (hybridization; VSEPR models; polarity; intermolecular forces) Lab Worksheet Lab #13 Activity Rubric Lab Worksheet Detecting Ions Complete Table Answer Questions (Due 1-31-13) Lab #12 Activity Rubric Lab Worksheet Flame Test Complete Table Answer Questions (Due 1-17-13) Lab #11 Write-Up (Due 1-14-13) Rubric Your Report Header (Emission Spectroscopy) Objective Procedure & Materials: Refer to Handout Data: Spectra attached Calculations: Write neatly in lab report (equations & units!) Discussion: Conclusion Questions; Errors/Improvements Claim – given on handout; Evidence (Spectra) Science Concept (electron energies, spectroscopy, continuous vs. line) Lab Worksheet Lab #10 Write-Up Rubric Your Report Header (The Periodic Table) Objective Procedure: Refer to handout Data: Refer to attached tables and graphs Discussion: Questions (Type in report; include question) Errors/Improvements (omit) Conclusion (Due 1-17-13) Claim – given on handout; Evidence (Graphs – state relationships and point to graphs as evidence of that observation.) Science Concept (ion formation; metals vs. nonmetals, etc.) Lab Worksheet Lab #9 Write-Up Rubric Your Report Header (Effect of Pressure on Volume of a Gas) Objective Procedure & Materials: Refer to Handout Data: Refer to Table in Handout Graphs: Volume vs. Pressure (two graphs with all requirements) Discussion: Questions (called Prelab Questions) Errors/Improvements Conclusion (Due 12-4-12) Claim; Evidence (refer to graph and PV=k relationship) Science Concept (density and PV, properties of gases, etc.) Lab Worksheet & Data Sheet attached Lab #8 Write-Up Rubric Your Report Header (Measuring Specific Heat) Objective Procedure and Materials: Refer to Handout Data: Organize your data in a table. Calculations: Show equation, sub w/units; answer w/units. Include heat lost by metal, gained by water; specific heat calculation, % error (make sure to state heat lost metal = heat gained by water). Discussion: Questions (Write or restate questions) Errors/Improvements (explain experimentally!) Conclusion (Due 11-5-11) Claim (given on handout) Evidence (Data table; cite examples) Science Concept (Calorimetry, specific heat, conservation of energy) Lab WS followed by Lab Data Sheet Lab #7 Write-Up (Due 10-23-11) Rubric Your Report Header (Physical and Chemical Changes) Objective Procedure and Materials: Refer to Handout Data: Include OBSERVATION TABLE in report Discussion: State evidence for chemical reaction; describe physical changes Questions (Analysis); Errors/Improvements Conclusion Claim (given on handout) Evidence (Observation table; cite examples) Science Concept (Physical/Chemical changes; Evidence of chemical reactions; Dissolving as a physical change) Lab WS followed by Lab Data Sheet Lab #6 Activity (Due 10-16-11) Rubric Lab WS: Classifying Matter Completed Table Lab #5 Write-Up (Due 10-16-12) Rubric Your Report Header (Simulating Half-Life) Objective Procedure and Materials: Refer to Handout Data: Table attached. Graph: Average # Pennies vs. Half-Life Discussion: Conclusion Questions (WS); Errors/Improvements Claim (Half-Life can be simulated by probability) Evidence (Graph relationship; decay; no zero level) Science Concept (Define half-life; Extension – what isotope has the longest half-life? The shortest?) Lab WS followed by Lab Data Sheet Lab #4 Write-Up (Due 10-10-12) Rubric Your Report Header (Radioactive Decay Cards) Objective: Determine and illustrate the radioactive decay of U-238. Procedure & Materials: Refer to Handout Data Discussion: Type question then answer in report or answer by restating the question. Errors/Improvements Conclusion Table: List of Nuclear Equations Graph: Mass Number vs. Atomic Number Claim; Evidence (Refer to the graph for illustration of decay series) Science Concept (Cause and consequence of unstable nuclei; n/p ratio for stability; define radioactivity; describe radioactive particles and dangers; etc.) Lab Worksheet Lab #2 Write-Up (Due 10-2-11) Rubric Your Report Header (Atomic Mass of Beanium) Objective Procedure and Materials: Refer to Handout Data/Graph: See data table on worksheet Calculations: Average Atomic Mass: Method 1 vs. Method 2 Discussion: Conclusion Questions (WS); Errors and Improvements Claim; Evidence; Science Concept (meaning of “average” atomic mass; refer to calc. in table; define isotope; elements are mixtures; etc.) Lab WS followed by Lab Data Sheet Lab #2 Activity Rubric Lab Worksheet (Due 9-27-12) Drawing Atomic Diagrams Complete Table Diagrams should show: Nucleus with p+= ___ and no= ___ and highlighted in colored pencil. Energy levels with # electrons labeled as eo =___ Lab #1 Activity Rubric Lab WS: Calibration Completed Table (Due 9-17-12) Outlines for Lab Write-ups Honors Chemistry 2012-2013