HO HO OH! - Science Horizons Initiative
advertisement

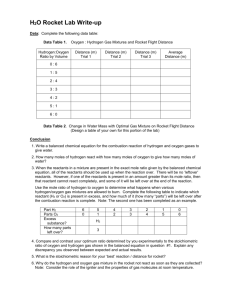
HO HO OH! A Hands On Investigation Of Hydrogen and Oxygen And their OH! (WOW) Properties. Presenter: Justin Guyer jguyer@lewistown.k12.mt.us Fergus High School SHI 2010 RATIONAL • Hands-On Activity/High Student Interest • Multiple science concepts: – – – – – – Properties of matter and measurement Atoms, molecules, and ions Properties of gases Stoichiometry Thermochemistry Scientific Method • Apply lab equipment, lab technique, and lab safety • Portfolio of 36 Labs to compliment High School Chemistry course Montana Standards • Content Standard 1 – Students, through the inquiry process, demonstrate the ability to design, conduct, evaluate, and communicate results an reasonable conclusions of scientific investigations. • Content Standard 2 – Students, through the inquiry process, demonstrate knowledge of properties, forms, changes and interactions of physical and chemical systems. • Content Standard 6 – Students understand historical developments in science and technology. General Instructional Goals • Students will be able to: – Collect and test for hydrogen and oxygen gas – Understand the principles of combustion reactions, exothermic and endothermic reactions, stoichiometry of reactions. – Launch a simple rocket that uses H2 and O2 gases. • explore and determine the proportions of hydrogen and oxygen mixture that will achieve the best launch results. • compare the balanced chemical reaction of hydrogen and oxygen with their lab results; – Perform the lab, collect data, and discuss, compare, and contrast their lab findings . Topic Sequence • Electrolysis of water • Methods of collecting Hydrogen gas – Properties of hydrogen gas • Methods of collecting Oxygen gas – Properties of oxygen gas • Scientific process of testing the best hydrogen/oxygen mixture to propel rocket Materials • Electrolysis apparatus • • Tesla coil • • Hydrogen peroxide 30% • • Manganese dioxide • • Power supply • • Plastic pipettes • • Zinc or magnesium Hydrochloric acid Test tubes w/1 hole stoppers Glass tubes Syringes 12-gauge copper wire Water trough and gas collecting apparatus Individual lesson Plans • Day 1 Electrolysis of water – Classification of matter, properties of gases, chemical reaction, acidity? • Day 2 Generation and collection of H2 – Properties of gas, thermochemistry, stoichiometry, gas laws, chemical reaction • Day 3 Generation and collection of O2 – Properties of gas, thermochemistry, stoichiometry, gas laws, chemical reaction • Day 4 & 5 Process of building and testing rockets Launching the Rockets 1. Cut the end off of a pipette leaving about 2 cm of stem attached to the bulb. Place 4 equal marks on bulb of pipette with permanent marker for ratio use. 2. Immerse the pipette in a pan of water and completely fill the "rocket" with water. 3. Slowly displace the water with the hydrogen and/or oxygen gases .(Best method-test tube, glass tubing?) 4. Slip the pipette rocket over a copper wire (12-14 gauge) that is grounded to Earth until the ends of the copper wire are above the water in the gas-filled region of the pipette. 5. With some water in the stem, launch the rocket by triggering a spark with tesla coil. 6. Vary the activity by changing the ratios of gas mixtures, and or try cutting the tips of the pipettes to two or three different lengths. Learner Outcomes • • • • • • • • • • • Balancing and type of chemical equations Stoichiometry Gas Laws PV=nRT Error analysis Physical and chemical properties of hydrogen and oxygen gas Understanding of exothermic vs endothermic Newton’s laws of motion Vocabulary development Lab technique and tool usage Scientific method Variables General Objectives/Assessment • Capstone Project – Since all concepts will have been learned prior to doing this unit, students will generate a detailed lab report incorporating concepts mentioned before. Assessment of SHI Project • Ways to determine success of Project – Increase of Student scores on tests and quizzes – Capstone project – Formal Assessment of student understanding of vocabulary and concepts while doing the lab. – Continued increase of students taking chemistry and AP Chemistry – HO HO OH!