Osmosis Investigation Lab Purpose: To demonstrate the 3
advertisement

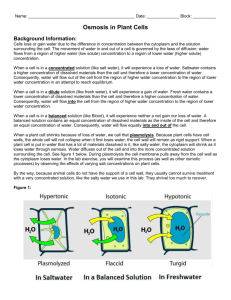
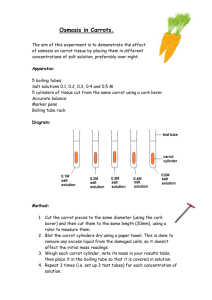
Osmosis Investigation Lab Purpose: To demonstrate the 3 conditions of Osmosis using carrots and celery Pre-Lab Questions: 1. What type of transport is Osmosis? 2. What direction does Osmosis move along the concentration gradient? 3. Does Osmosis require ATP? 4. Define Hypotonic 5. Define Hypertonic 6. Define Isotonic 7. What kind of cells make up carrots and celery? Materials: Ziploc 3 carrots 10% NaCl solution 3 celery Distilled water Tap water Procedures: 1. Obtain 3 ziploc baggies and label each with Distilled, 10 % NaCl, and Tap water 2. Label the baggies with your table number and class period (ex: 1-1 table 1, class 1) 3. Obtain 3-carrots and 3- celery 4. Get the mass of each and record in data table 5. Pour a sample of each solution in the appropriately labeled baggie (enough to cover the carrot and celery) 6. Seal the baggie so that as little air as possible is left in the bag 7. Next day record the mass of each carrot and celery in the appropriate data table 8. Answer analysis questions 9. Hypothesis: (Predict the type of water will keep the carrot and celery crisp) _________________________________________________________ _________________________________________________________ ________________________________________________________ Data table 1: Initial Mass of Carrot and Celery Vegetable Carrot Distilled __________(g) 10% NaCl _________(g) Tap water __________(g) Celery __________(g) _________(g) __________(g) Data table 2: Final Mass of Carrot and Celery Vegetable Carrot Distilled __________(g) 10% NaCl _________(g) Tap water __________(g) Celery __________(g) _________(g) __________(g) Analysis Questions 1. What solution should Kroger’s use to keep their vegetables fresh? _________________________ 2. What evidence do you have to support your claim? ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ 3. Which solution was Hypertonic? _____________________________________ 4. Which solution was Hypotonic? __________________________________________________________________ 5. What information would you need to know to make an Isotonic solution? ______________________________________________________________ ______________________________________________________________