ISAT 420 - Exercise

2015
Industrial Facility X
INORGANIC PHOSPHOROUS EMISSION AND LONG TERM EFFECTS
MATTTHEW MCCARTER AND MAX DECKER
Table of Contents
1 | P a g e
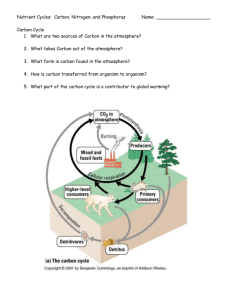
1 Introduction
The model is a representation of Industrial Facility X located on a lake and contributes a continuous, but not necessarily a constant, flow of inorganic phosphorus into the lake. The goal is to aid an environmental regulator in decisions regarding potential regulations on Industrial Facility X through investigating the facilities output of inorganic phosphorus into the lake and the effects on the overall balance of the occurring phosphorus cycle of the lake.
2 System Boundaries
There are two boundaries inside the system that consists of (1) the Phosphorus Cycle within the lake and
(2) the BOD/DO relationship within the lake. These boundaries were set to have initial steady state cycles for each of the boundaries. This was what was chosen to accurately investigate the input of effluent without concern for other influences.
3 Flux
Our flux consists of inorganic Phosphorous levels emitted from Industrial Facility X into the phosphorus cycle within the lake and levels of dead organic phosphorous into algae blooms.
4 Time Frame
The time frame for the model is twenty years. Twenty years is the time frame because it will give enough time to get an accurate representation of the short term to beginnings of long term effects.
5 Temporal and Spatial Resolution
The temporal resolution for our model was one-tenth of a year, and this was chosen as a resolution that gives us a good representation of the data. Spatial resolution was the size of the Lake, which was 10^12
Liters.
6 Scope
Included in our model was phosphorus Levels within the lake, organic and inorganic. Also included were
BOD, DO, and Algae levels within the lake. Finally included is the inorganic phosphorous levels coming from nearby Industrial Facility X. Exclusions include outside inorganic phosphorous sources other than
Industrial Facility X.
7 Assumptions
Steady-state phosphorus cycle
Steady-state BOD/DO relationship
Industrial Facility X outflow levels are continuous but not constant
No outside P sources other than industrial facility
No outside BOD source other than algae blooms
No outside sources of DO
Continuous flow of phosphorous from industrial facility
Not constant levels of flow from the facility
All phosphorous from the facility enters the lake, non is lost to surroundings
Lake Volume is 10^6 Liters
Assumed starting values for parameters. These
will be discussed in later section
Phosphorus is limiting factor
Nitrogen is constant
Randomized Inflow of Inorganic Phosphorus ±
200 mg, from a value (4815 mg/0.1 year) retrieved from online sources. (Qasim 1985)
2 | P a g e
8 The System
8.1
Flow diagram and Pic of Stella Model
Figure 8.1.1: Shows the total flows into and out of the boundaries within our system model.
8.2
Phosphorus Cycle
Figure 8.1.2: Shows the Phosphorus cycle boundary within the system. Industrial Facility X produces a continuous but not constant flow of inorganic phosphorus.
8.3
BOD/DO Relationship
Figure 8.1.3: Shows the BOD/DO relationship within the systems model. As BOD increases we expect to see DO decrease
1 | P a g e
8.4
Nitrogen Inflow:
Figure 8.1.4: Shows the Nitrogen inflow and the pairing of available phosphorus, being the limiting factor and nitrogen in the system. It is not a one-to-one ratio and that ratio will be discussed in later sections.
8.5
Stella Model
Figure 8.1.5: Shows the linkage used in the actually creation of the model using the Stella program
2 | P a g e
9 Enumerate Relationships
9.1
Types of Relationships
Donor Controlled – the flow of matter equals some rate constant times the amount of matter in the donor stock (Deaton 2000)
Receptor Controlled – the flow of matter equals some rate constant times the amount of matter in the receptor stock (Deaton 2000)
Donor and Receptor Controlled – high levels of either donor or receptor stock variable will lead to high flows of matter between these two stocks. (Deaton 2000)
9.2
Mathematical Equations Used
Living to Dead Matter Flow
“a” is a rate constant
𝐷𝑒𝑎𝑡ℎ = 𝑎 × 𝐿𝑖𝑣𝑖𝑛𝑔 𝑂𝑟𝑔𝑎𝑛𝑖𝑐 𝑃ℎ𝑜𝑠𝑝ℎ𝑜𝑟𝑢𝑠
Flow of matter from a living organic state to a dead organic state is usually a donor-
controlled process. We expect that the amount of living matter will control the flow from living to dead, unless the existence of the dead organisms actually affects factors that may cause death. (Deaton 2000)
Inorganic to Living Matter Flow
“c” is a rate constant
𝑈𝑝𝑡𝑎𝑘𝑒 = 𝑐 × 𝐿𝑖𝑣𝑖𝑛𝑔 𝑂𝑟𝑔𝑎𝑛𝑖𝑐 𝑃ℎ𝑜𝑠𝑝ℎ𝑜𝑟𝑢𝑠 × 𝐼𝑛𝑜𝑟𝑔𝑎𝑛𝑖𝑐 𝑃ℎ𝑜𝑠𝑝ℎ𝑜𝑟𝑢𝑠
Flow of matter representing the uptake of inorganic nutrients by a living organic state is usually a donor- and receptor-controlled process. Both the amount of nutrients available and the number of living organisms vying for those nutrients dictate this flow. (Deaton 2000)
Dead to Inorganic Matter Flow
“b” is a rate constant
𝐷𝑒𝑐𝑜𝑚𝑝 = 𝑏 × 𝐷 ead Organic Phosphorus
Flow of matter from a dead organic state to an inorganic state (decomposition) is usually a donor-controlled process. We expect the flow to be driven by the amount of dead matter that is decomposing and not by the inorganic matter to which the dead matter is flowing.
(Deaton 2000)
Phosphorus Inflow
𝑃ℎ𝑜𝑠𝑝ℎ𝑜𝑟𝑢𝑠 = 1 + 𝐼norganic Phosphorus
Phosphorus inflow is equal to the Inorganic Phosphorus due to Inorganic Phosphorus being the type of Phosphorus contributing to the increase in BOD levels. The “1+” is used to the initial state of the system being steady-state, inflows equaling outflows (1=1).
Biologic Oxygen Demand Inflow
𝐵𝑂𝐷 𝐼𝑛𝑝𝑢𝑡 = 1 +
𝑃ℎ𝑜𝑠𝑝ℎ𝑜𝑟𝑢𝑠+(0.1×𝑁𝑖𝑡𝑟𝑜𝑔𝑒𝑛)
2
BOD inflow calculations was a ratio of P=0.1N. This ratio was used due to the abundance of
Nitrogen in the system at the start and the initial lack of Phosphorus compared to the initial levels of Nitrogen.
3 | P a g e
Decomposition of Biologic
K
1 is the exponential decay constant
𝐵𝑂𝐷 𝑂𝑢𝑡𝑝𝑢𝑡 = 𝐵𝑂𝐷 × 𝑘
1
Equation representing the natural decay/decomposition of biological matter
Deoxygenation
𝐷𝑂 𝑂𝑢𝑡𝑝𝑢𝑡 = 𝑘
1
× 𝐵𝑂𝐷
Equation representing the natural deoxygenation of dissolved oxygen through use and escape into the atmosphere.
Re-oxygenation
K
2 is the re-oxygenation coefficient
DO Saturation is the theoretical saturation level of the water, or the max amount of dissolved oxygen the water can hold
𝐷𝑂 𝐼𝑛𝑝𝑢𝑡 = 𝑘
2
× (𝐷𝑂 𝑆𝑎𝑡𝑢𝑟𝑎𝑡𝑖𝑜𝑛 − 𝐷𝑂)
Equation representing the DO Saturation value and how when the oxygen deficit (DOsat –
DO) is high then the re-oxygenation rate should increase.
9.3
Steady-State Model Run
9.3.1
BOD/DO Relationship Results
Figures 9.3.1 Depicts to typically outcome for our trial runs using the initial steady-state values and a base p=0.1N BOD inflow ratio, and a varying value for effluent inflow of 4015 mg/yr ± 200 . We expected as the BOD increased the DO would decrease, but only to the point where Phosphorus is not the limiting factor.
Figure 9.3.1: Depicts the typically outcome for BOD/DO values in our trial runs using the initial steady-state values, a base p=0.1N BOD inflow ratio, and a randomized value for effluent inflow of 4015 mg/0.1yr ± 200.
4 | P a g e
9.3.2
Phosphorus/Nitrogen Pair Results
Figure 9.3.2: Depicts the typically outcome for the P/N values in our trial runs using the initial steady-state values, a base p=0.1N BOD inflow ratio, and a randomized value for effluent inflow of 4015 mg/0.1yr ± 200.
10 Relationships
10.1
Phosphorous Cycle
The Phosphorus Cycle is a balance of three types of phosphorus: living organic, dead organic, and inorganic phosphorus within the lake. The cycle within our model was based on the cycle given from
Dynamic Modeling of Environmental Systems (Deaton 136)
10.2
P and N pairs to BOD
In modeling our system we assumed that initially Nitrogen levels in the lake are significantly greater than the available phosphorus, making phosphorus the limiting factor when considering how increased phosphorus increases BOD levels.
It takes Nitrogen/Phosphorus pairs to increase BOD, and to demonstrate the limiting factor a ratio of phosphorus to Nitrogen was used. Initially available phosphorus only accounted for one-tenth of nitrogen due to the abundance of Nitrogen in the system. For example for every 0.1 P 0.1 N is paired, so
0.9 N goes unpaired/unused.
10.3
BOD/DO Relationship
The BOD/DO relationship was based off of the Streeter Phelps Model, but due to lack of some information the initial state of this relationship in our model is a steady-state where the inflows equal the outflows for BOD and DO.
5 | P a g e
This steady-state initial was chosen because the goal was to investigate the changing in the levels due to an inflow of inorganic phosphorus. Incorporating a DO Sag curve was not deemed necessary, but in the future could be an improvement made to this model.
11 Influence
Changes in the facilities output levels can influence the model. Examples would be overproduction
(assuming more production = more outflow), implementing any phosphorus management techniques reducing the P levels, and variations in day to day outflow. Any other potential influences were excluded to focus on the facilities impacts.
12 Determine the units for variables and parameters/coefficients
12.1
Parameters and Units Used
These units were used mainly due to the given assumed values, and the frequency of these units when comparing Phosphorus, BOD, DO, and Volume.
DO levels – mg/L
BOD levels – mg/L
Phosphorus levels – moles/L
Lake Volume – L
13 Calibrate
Independent variables are only the phosphorus levels in the outflow from Industrial Facility X, other potential variable begin in a steady state and their change is dependent on change in the IV, phosphorus levels in the outflow. The dependent variables are the DO levels within the lake.
Due to the specific nature of our hypothetical scenario it was difficult to have data for calibration all from a singular source. Using data gather from multiple sources we retrieved values for initial phosphorus and nitrogen levels (Thomas 2015), and effluent levels coming from Industrial Facility X
(Qasim 1985).
13.1
Calibration Model Run
These runs were performed using values for Nitrogen and Phosphorus initial and Effluent Levels retrieved from previously discussed sources. The values being N=1.489 mg/l, P=0.06 mg/L, and a varying effluent level of 4015 mg/0.1yr ± 200.
6 | P a g e
13.1.1
BOD/DO Relationship Results
Figure 9.3.2: Depicts the typically outcome for the P/N values in our trial runs using values previously discussed.
13.1.2
BOD/DO and Phosphorus/Nitrogen Pair Results
Figure 9.3.2: Depicts the typically outcome for the BOD/DO values in our trial runs using values previously discussed.
7 | P a g e
14 Conclusion
We expected BOD levels to increase in the lake’s natural system when inorganic phosphorus is added from industrial facility X up to the point when phosphorus is not the limiting factor, P>N. The model successfully represented a BOD increase when inorganic phosphorus from industrial facility X was introduced into the lake system. Phosphorus was the limiting factor until it exceeded the nitrogen level; at this point nitrogen becomes the limiting factor. Once nitrogen became the limiting factor BOD no longer increased. This information is valuable and attains the goal of aiding an environmental regulator in administrating regulations regarding Industrial Facility X’s inorganic phosphorus output into the lake.
The decision to impose regulation on Industrial Facility X would be based on comparing the BOD values modeled to State environmental regulation levels for the location of Industrial Facility X. Effects of concern when comparing the BOD levels would be ecological impacts and human health risks if the waterbody sources a drinking water system.
8 | P a g e
15 References
1.
Deaton, M., & Winebrake, J. (2000). Dynamic modeling of environmental systems (pp. 113 -
141). New York: Springer.
2.
Thomas, C., Puccio, M., & Johnson, D. (2013). Smith Mountain Lake Water Quality Monitoring
Program. Retrieved November 18, 2015, from http://www.ferrum.edu/academics/schools/natural_sciences_math/projects/sml_water_quality
/yearly_reports/2013SMLReport.pdf
3.
Qasim, S. (1985). Wastewater treatment plants: Planning design and operation (Second ed., p.
94). New York: Holt, Rinehart, and Winston.
9 | P a g e