PPT - Weizmann Institute of Science
advertisement

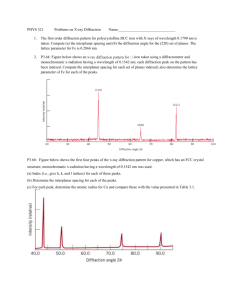
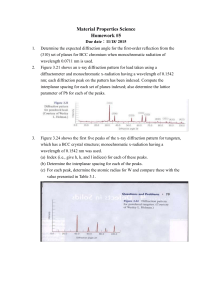
Heavy Atom Quantum Diffraction by Scattering from Surfaces Eli Pollak Chemical Physics Department Weizmann Institute of Science Coworkers: Dr. Jeremy M. Moix Grants: Israel Science Foundation Weizmann-UK Joint Research Program http://www.ams.org/featurecolumn/archive/rainbows.html Bill Casselman University of British Columbia, Vancouver, Canada cass at math.ubc.ca qf b 4r n·sin(qr)= sin(qi)=b/R=x, R n=4/3 qf(x) = 4qr-2qi = 4sin-1(x/n)-2sin-1(x) dqf/dx=0 => x=√(20/27), qf=42o The rainbow angular distribution: 1 x P (q ) = dx q 4 sin 1 2 sin 1 x ) 2 1 n 1 Water: n=4/3 Perspex: n=3/2 Rainbow scattering from a hard wall h(x) p/2fqi f f=tan-1[h’(x)]~-h’(x) qf (x)=qi+2f~qi2h’(x) qi p/2f qf p/2fqi x l d 2 h( x ) The rainbow angles are found when = 0 they arise from dx 2 the deflection points of the corrugation function. The angular distribution for scattering from a hard wall l 1 p q ) = dx q q i 2h' ( x ) ) l0 2px If the corrugation is sinusoidal: h ( x ) = h sin l then the angular distribution is readily seen to be: 1 4ph 1 2px p (q ) = dx q q i cos = 2 l0 l l 4ph 2 ) q q i l l l=30 mm h=0.42 mm qR=qi±10o Experimental observations I. In plane scattering of Ar atoms on a LiF(001) surface: Note: At 100 meV the de Broglie wavelength of Ar is 0.25 a.u.. The lattice length is ~ 8 a.u.. It is then not surprising that the measured distribution is classical. II. In plane scattering of Ar and Kr on a Ag(100) surface Ar Kr III. In plane scattering of Ar on a 2H-W(100) surface At 100 meV the de Broglie wavelength is 0.25 a.u.. Why then does one see diffraction at Ei=27 meV? (The lattice length is ~ 10 a.u..) Beam energy uncertainty of 1/30 implies a parallel coherence wavelength of ~7.5 a.u., enough to see the diffraction peak. Krypton mass - ~84, at 35 meV, dBwl~0.3 a.u.. Beam energy uncertainty of 1/35 implies a parallel coherence wavelength of ~10 a.u., enough to see the diffraction peak. Heavy molecule interference experiments: Two slit diffraction experiment Waves at the two slits must be coherent Fullerene speed of 100 m/s → 5.6 pico m de Broglie wavelength Collimating the beam with 7 mm slits separated by 1 m implies that transverse speed is less than 1.4·10-3 m/sec → ~400 nm de Broglie wavelength or an angular width of ~8 10-4 degrees. Note the r2 dependent loss of signal due to the long path between the collimating slits. High energy grazing collisions Diffraction of Fast Atomic Projectiles during Grazing Scattering from a LiF(001) Surface PRL 98, 016103 (2007) A. Schueller, S. Wethekam, and H. Winter* Institut fuer Physik, Humboldt Universita¨t zu Berlin, Brook-Taylor-Strasse 6, D-12489 BerlinAdlershof, Germany Light atoms and molecules with energies from 300 eV to 25 keV are scattered under a grazing angle of incidence from a LiF(001) surface…. Experimental results for scattering of H, D, 3He, and 4He atoms as well as H2 and D2 molecules can be unequivocally referred to atom diffraction with de Broglie wavelengths as low as about 0.001 A° Analysis: At 10 keV the de Broglie wavelength of 4He is 0.0027 a.u. a 1o grazing angle implies p┴~p║/60 so a perpendicular wavelength of 0.16 a.u.. The angular resolution is ~0.01o so that the coherence wavelength is ~16 a.u., enough to observe diffraction. Theory – A typical diffraction pattern A square surface A hexagonal surface At low energy quantum mechanics prevails Consider again the Ar-LiF scattering experiment: Classical and quantum scattering on frozen (model) surface. Ar energy of 705 meV → 5.5 pm de Broglie wavelength while LiF lattice length =400 pm. The angular width of the beam is 2 degrees so the perpendicular coherence wavelength is ~ 180 pm. Classical Wigner Exact quantum Reducing the uncertainty in the transverse momentum so that the angular width of the incident beam is 0.2o leads to the quantum mechanical diffraction pattern: The typical lattice length is 1 nm, a factor of 100 smaller than the size of the grating used in the two slit experiment, implying a reduction of the collimation length by 102 and thus a gain of 104 in the signal to noise ratio. The final momentum distribution with an angular incident width of 0.2o Decoherence via increase of surface temperature Phonons modeled as single oscillator coupled to the vertical motion Preliminary experimental results – scattering of Ar on Ru(0001) M. Minniti and D. Farias, Madrid. Energy – 64 meV, de Broglie wavelength – 18 pm. Lattice length – 300 pm Coherence length – 180 pm (2 mm aperture) 400 pm (.75 mm aperture) 720 pm (0.4 mm aperture) Summary: • Quantum diffraction of heavy atoms is observable in surface scattering at low temperatures. • Collimation of the incident beam leads to partial diffraction patterns. • At sufficiently low surface temperature, surface phonons do not wipe out the coherence • Decoherence may be studied quantitatively by varying the surface temperature Future directions • Use better theory (MCTDH) to include a realistic representation for the continuum of phonon modes. • Study diffraction of molecular systems – do internal modes destroy the quantum diffraction through energy exchange? • Can heavy atom diffraction be used to study surface interactions – phonons or electronic? • Is it possible to use the quantum coherence to manipulate energy transfer with the surface? • Can one conehrently control reactive processes on the surface? Energy loss rainbows J. Moix, E. Pollak and S. Miret-Artès, Phys. Rev. Lett. 104, 116103 (2010). From the perturbation theory we found the Gaussian energy transfer kernel and the energy distribution of the scattered particle: Consider then the energy loss as a function of impact parameter for Ar-LiF scattering: Joint energy and angular distribution for a 15O angle of incidence, 3eV incidence energy and surface temperature T=30K. Have they been seen experimentally? Rotational Rainbows C2=0.01, C1=0 J = 0 = 1, I 2 2 E Z = M 2 For simplicity consider a diatomic molecule with angular momentum parallel to the surface. Let qi denote the initial angle of the diatomic axis relative to the vertical to the surface: q For a smooth surface, the interaction potential will be a function of the angle q. The final angular momentum will then depend on the initial rotational angle. Perturbation theory The Hamiltonian: 2 p p p 1 2 ' V1 Z , q q ' , ')) H= V Z ) pq ' 2 2m A m B ) 2I sin q ') 2 X 2 Z The unperturbed motion: pq ' = 0, p ' = J 0 , q '= p 2 1st order perturbation theory: , ' t ) = ' t 0 ) J0 t t 0 ) I pq ' = pq ' V1 ) J 02pq ' J 0 ) p ' = p ' V1 ) Energy conservation: ) PZ 0PZ 1 2 2 = 2 J 0p ' V1 ) p ' V1 ) pq ' V1 ) = E rot m A m B ) 2 I The angular distribution: tan i = PX 0 , PZ 0 tan f sin 2 i ) Erot 4 EZ PX 0 PZ 1 PZ 0 PZ 0 When the molecule is not too slowly rotating one readily finds: P ) = 1 p r2 sin 4 q J ) 2 → The rotational rainbows cause maxima in the angular distribution! Examples: V Z ) = V0 e 2Z 2e Z ) → Morse potential 3 Z V1 Z , q ) = V0 2C1e 1 cosq )) C 2 e 2Z sin 2 q ) 2 (C1=0 for a homonuclear diatomic)