Percent Water in a Hydrate Lab: Chemistry Experiment
advertisement

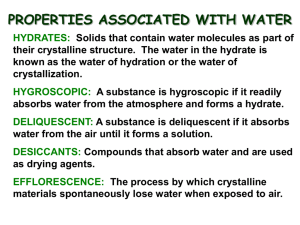
LAB # 3.5 Lab: Percent Water in a Hydrate Objectives Analyze the percent water in a crystalline hydrate Identify the hydrate from a list of possible unknowns Introduction A compound is a pure substance - it has a fixed (constant) composition. The composition of a pure substance is the same throughout and does not vary from one sample to another. According to the law of definite proportions, a compound always contains the same elements in the same proportions, regardless of the amount of the sample, where it was found, or how it was prepared. A mixture, on the other hand, may contain variable amounts of different substances. The composition of a mixture is not constant. A hydrate is a pure substance that contains water molecules embedded in its crystal structure. Heating a hydrate "drives off" the water molecules, and the solid that remains behind is called anhydrous, meaning "without water." The chemical formula of a hydrate specifies the relative number of each kind of atom in a formula unit of the compound, as well as the number of water molecules bound to each formula unit. Calcium chloride dihydrate, which is used as road salt, is an example of a hydrate. The chemical formula for calcium chloride dihydrate is CaCl2•2H2O. The "dot" in the chemical formula indicates that two water molecules (H2O) are attached or bound to the ions in solid calcium chloride (CaCl2) by which chemical bonds. The water in calcium chloride dihydrate can be removed by heating the hydrate (Equation 1). heat CaCl2•2H2O 147.02 g dihydrate → CaCl2 110.98 g anhydrous salt + 2H2O Equation 1 36.04 g The number of water molecules in a hydrate is called the water of hydration. The water of hydration of calcium chloride dihydrate is two water molecules per every one formula unit of calcium chloride. The number of water molecules in a typical hydrate is characteristic of that particular salt and is usually a small whole number from 1 to 10. The chemical formula of a hydrate can be determined by analyzing the percent water in the hydrate - the ratio of the mass of the water of hydration lost upon heating divided by the mass of the original hydrate (Equation 2). mass of water of hydration x 100 = percent water in hydrate Equation 2 mass of hydrate 1 Introduction Continued: The formulas of some common hydrates and their anhydrous salts are summarized in Table 1. Table 1. Formulas of Hydrates and their Anhydrous Salts Common Chemical Hydrate name name Washing soda Gypsum Epsom Salt Sodium carbonate monohydrate Calcium sulfate dihydrate Magnesium sulfate heptahydrate Na2CO3•H2O CaSO4•2H2O MgSO4•7H2O Anhydrous Salt Na2CO3 CaSO4 MgSO4 Materials Chemical-resistant goggles, apron, and gloves Chemical scoop Distilled water Electronic gram balance Laboratory burner and tubing Ring stand Support stand Sparker Test tube (Pyrex®), medium Test tube clamp, uncoated "Unknown" crystalline hydrate Weigh boat Wire gauze or heat-resistant ceramic pad Safety Precautions Recall the general safety rules for working with a laboratory burner. Keep long hair tied back, make sure that hair, clothing and hands are a safe distance from the flames at all times, and always wear goggles and closedtoe shoes in case of spattering. Never reach over the top of a burner flame. Light the burner only as instructed by the teacher and do not leave a lit burner unattended. Always turn the burner off when not in use. Use only heat-resistant, borosilicate glassware(e.g., Pyrex®). Heated materials remain hot for a very long time, and HOT GLASS LOOKS EXACTLY THE SAME AS COLD GLASS. Place the test tube on a wire gauze or on a heat-resistant pad to cool. The crystalline hydrates used in this lab are slightly toxic by ingestion and may be irritating to skin and eyes. Avoid contact of all chemicals with eyes and skin. Wear chemical-splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soap and water before leaving the lab. Answer the pre-lab questions found at the end of this lab packet. Tear off the answer sheet and turn it in by: _________ 2 Procedure 1. Observe the *color and appearance of the unknown hydrate and record the observations, along with the label code, in the data table on the Post Lab section. 2. Set up a support stand with an uncoated test tube clamp. 3. Adjust the height of the test tube clamp so that a laboratory burner can be moved freely under the test tube during heating (step 8). See Figure 1. 4. Measure the mass of a clean, dry test tube to the nearest 0.001 g. Record the mass in the data table in the Post Lab section. 5. Use a chemical scoop to transfer about 2 grams of unknown crystalline hydrate to the test tube. 6. Mass the combined mass of the test tube and the unknown crystalline hydrate. Record this mass in the data table in the Post Lab section. 7. Hold the test tube almost horizontally and GENTLY tap the test tube until the hydrate is spread out over the bottom one-third portion of the test tube (Figure 1). Note: Some hydrates will spatter when they are heated. 8. Insert the test tube into the test tube clamp being careful to keep the test tube almost horizontal with the open end slightly elevated. 9. Attach the test tube clamp to the ring stand again being careful to keep the test tube almost horizontal with the open end slightly elevated (Figure 1). 10. Light the laboratory burner and "sweep" the bottom of the test tube with the top of the burner flame. Continue to sweep the flame along the portion of the test tube containing the hydrate for about 4-5 minutes. 11. Periodically brush the flame over the top and mouth of the test tube as well to evaporate any water droplets that collect there. 12. Turn off the burner flame when there is no more water vapor escaping from the opening of the test tube and no further changes are observed in the appearance of the test tube contents. Note: For most hydrates, 5 minutes of heating (steps 10 and 11) will be sufficient. However, for a few hydrates, up to 15 minutes of heating will be required. * examples: white large crystals, or blue powder, or yellow small crystals, or orange granular 3 Procedure Continued: 13. Allow the test tube to cool completely for 8-10 minutes. Note: The test tube is cool enough to weigh if you do not feel any radiant heat when placing your hands above the test tube. 14. Measure the combined mass of the test tube and the anhydrous residue. Record this mass in the data table in the Post Lab section. 15. Observe the color and appearance of the anhydrous residue and record the observations in the data table in the Post Lab section. Clean Up Dispose of the contents of the test tube into one of waste disposal containers in the fume hood. DO NOT throw the residue in the trash can!!! 4 Name __________________________________ Date ____________________ Period ______ POST-LAB QUESTIONS for LAB 3.5: Percent Water in a Hydrate Data Table: "Unknown" hydrate label Code(Step 1) Color and Appearance of unknown Hydrate (Step 1) Mass of test tube (Step 4) Mass of test tube and hydrate (Step 6) Mass of test tube and anhydrous residue (Step 14) Color and Appearance of anhydrous residue (Step 15) 1. Calculate the following. Follow ALL math work rules! (a) the original mass of the hydrate: (b) The mass of the water of hydration: (c) the percent water in the hydrate: 5 Lab #3.5 Post-Lab Question Continued: 2. The unknown hydrate is one of the following substances. Complete the table to calculate the theoretical percent water for each hydrate. You do NOT need to show any work. 3. CuSO5•5H2O MgSO4•7H2O BaCl2 •2H2O Sum of atomic masses (anhydrous salt) 159.61 120.38 208.23 Sum of atomic masses (nH2O) 90.10 Sum of atomic masses (hydrate) Percent of water in hydrate (theoretical) 4. (a) What is the probable identity of the unknown crystalline hydrate? (b) Explain your reasoning. 4. Assume you have correctly identified the unknown hydrate. Calculate your percent error in the percent water analysis. Use the following equation and follow ALL math work rules! theoretical value - experimental value percent error = x 100 theoretical value 6 Lab #3.5 Post-Lab Question Continued: 5. (a) Is the conversion of the hydrate to its anhydrous salt a physical or a chemical change? (b) Explain. 6. (a) Is the unknown hydrate a mixture or a pure substance? (b) Explain. 6. Consider the following potential sources of error in this experiment. ______ (a) water was observed around the mouth of the test tube after heating ______ (b) the anhydrous residue decomposed upon heating, liberating gas ______ (c) the hydrate spattered during heating and some of the sample was lost. 7 For each of the potential sources of error above indicate whether the percent water calculated in question 1(c) would be too high (H), too low (L), or unchanged (NC). Name __________________________________ Date ____________________ Period ______ PRE-LAB QUESTIONS for LAB 3.5 Percent Water in a Hydrate 1. State the reason that the glassware being used during a heating lab should always be assumed to be hot? 2. Compare the composition of a compound with that of a mixture. 3. a) According to the introduction to this lab, what is the law of definite proportions. b) Give a chemical example and explain WHY it obeys the law of definite proportions. 4. Define each of the following: 8 a) hydrate Lab # 3.5 Pre-Lab Questions Continued: 4. Contiued: Define each of the following: b) anhydrous salt c) water of hydration 5. Calculate the percent water in calcium chloride dihydrate. Follow ALL math work rules! 6. Briefly list three (3) general safety rules for working with a laboratory burner. 9 Lab # 3.5 Pre-Lab Questions Continued: 7. The following data were obtained when a sample of barium chloride hydrate was analyzed as described in the Procedure section. Mass of empty test tube Mass of test tube and hydrate (before heating) Mass of test tube and anhydrous salt (after heating) 18.42 g 20.75 g 20.41 g a) Calculate the mass of the hydrate. Follow ALL math work rules! b) Calculate the mass of the water of hydration. Follow ALL math work rules! c) Calculate the percent water in the hydrate. Follow ALL math work rules! 8. The general formula of barium chloride hydrate is BaCl2•nH2O, where n is the number of water molecules. Calculate the theoretical percent water for each value of n - divide the sum of the atomic masses due to the water molecules by the sum of all the atomic masses in the hydrate, and multiply the result by 100. Complete the table. You do NOT need to show any work - but make sure you do the work yourself so that you know HOW to do the work! BaCl2 BaCl2•H2O BaCl2•2H2O BaCl2•3H2O 208.23 208.23 208.23 208.23 0 18.02 Sum of atomic masses (BaCl2) Sum of atomic masses (nH2O) Sum of atomic masses (hydrate) 10 Percent water in hydrate(theoretical) 208.23 226.25 0% 7.96% Lab # 3.5 Pre-Lab Questions Continued: 9. Compare the percent water in the hydrate, question 7(c), with the theoretical values calculated for the different values of n in question 8. a) What is the most likely formula for barium chloride hydrate? ___________________________ b) Explain your choice. 10. TRUE / FALSE (circle one) 11. TRUE / FALSE (circle one) Since water of hydration is not liquid, there is no need to worry about spattering as the unknown crystalline hydrate is heated. Almost ALL hydrate must be heated for up to 15 minutes to completely evaporate all the water of hydration. 12. Explain how you will know that the heated test tube is cool enough to mass the anhydrous residue. 11