Anemia and Bleeding Point
advertisement

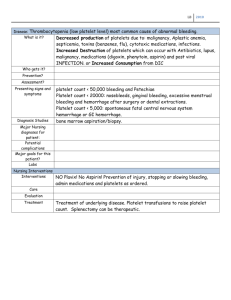
Anemia and The Bleeding Patient November 1, 2005 Eli Denney D.O. Definition of Anemia Anemia – a reduced concentration of red blood cells. Measured by Hct, Hgb and RBC count. Anything that reduces production or increases destruction will result in anemia if the processes are not corrected. Anemia - Causes Most common causes in U.S. Iron deficiency Thalassemia Anemia of Chronic Disease Physiologic Reactions to Blood Loss Acute – Peripheral vasoconstriction and central vasodilatation If blood loss continues – small vessel dilatation with compensatory decreased PVR, resulting in increased CO. Chronic - Increased plasma volume keeps intravascular volume normal Erythropoietin released by kidneys – reticulocytes in 3-7 days. Signs and Symptoms Depend upon Rate of blood loss Amount of blood lost Age Overall Health Comorbid disease states Signs and Symptoms Weakness Palpitations Fatigue Orthostatic Dyspnea symptoms Lethargy Physical Exam Findings + Orthostatic BP’s Widened pulse Tachycardia pressure GI bleeding/Uterine bleeding Altered Mental Status Pallor Systolic ejc. murmur Diagnosis Confirmed by lab values – RBC count, Hgb and Hct. CBC may not determine specific cause – need to obtain other labs to begin appropriate workup CBC-provides RBC indices (MCV) Reticulocyte count Peripheral smear Iron studies Folate, B12 levels Treatment In ED, anemia from hemorrhage is the most common need for treatment Symptomatic Hemodynamically unstable Asymptomatic - can do outpatient work up depends upon clinical situation. Based on clinical situation, patients should be typed and cross-matched for transfusion Admit Patients with ongoing blood loss for definitive care Increased Likelihood of Bleeding Disorder Spontaneous bleeding-multiple sites Bleeding from untraumatized sites Bleeding several hours after trauma Bleeding into deep tissues or joints Family history of bleeding disorder Excessive bleeding after dental extractions or surgical procedures Liver disease Increased Likelihood of Bleeding Disorder Drugs – Ethanol Coumadin ASA NSAIDS Antibiotics Plavix Bleeding Site May Indicate Specific Abnormalities Indicative of Platelet Disorder Mucocutaneous Bleeding Petechiae Ecchymosis Epistaxis GI/GU bleeding Heavy menstrual bleeding Purpura are associated with thrombocytopenia and commonly indicates a systemic disease Coagulation Factor Deficiencies Bleeding into joints, potential spaces, retroperitoneum and delayed bleeding Mucocutaneous bleeding and bleeding into deep spaces may be signs of DIC – both pathways of homeostasis are involved. Normal Coagulation Platelet Plug (Primary Homeostasis) Cross-linked Fibrin (Secondary Homeostasis) Fibrinolytic system – counter regulatory system that prevents excessive clot formation. Primary Homeostasis Depends upon platelet interaction with endothelium Requires normal vascular endothelium, functional platelets, von Willebrand factor, and normal fibrinogen von Willebrand factor connects platelets to the endothelium by glycoprotein Ia Fibrinogen connects platelets by glycoproteins IIB-IIIA. Secondary Homeostasis See Fig 218-3 - Coagulation Cascade Fibrinolytic System Purpose is to limit the size of fibrin clots Endothelial cells release tPA which converts plasminogen to plasmin which is already a part of the fibrin clot Plasmin degrades fibrinogen and fibrin monomer into fibrin degredation products and cross linked fibrin into D-Dimers Other Inhibitory Proteins Antithrombin III – inhibits clotting cascade. Inhibits function of XIIa, XIa, IXa and Thrombin. Heparin potentiates this interaction. Proteins C and S Activated Protein C – binds with cell surface bound Protein S – the bound proteins inhibit factors Va and VIIa. Inhibitory Proteins Deficiencies or dysfunction of proteins C,S or antithrombin III can cause a hypercoagulable state. Initial Testing for Bleeding Disorders CBC PT/INR PTT Further testing as indicated (Table 218-4) Acquired Bleeding Disorders Acquired Platelet Defects Quantitative Defects Decreased production Increased destruction Splenic sequestration Platelet levels less than 10-20,000 / microL increases the likelihood of spontaneous bleeding – especially intracranial Levels less than 10,000 – transfusion of platelets will be necessary Decreased Production Bone marrow infiltration Aplastic anemia Viral infections – CMV, Rubella Drugs (Thiazides, Ethanol, Chemo-agents) Vitamin B12, Folate deficiency Radiation Increased Destruction ITP Viral infections – TTP HIV, Varicella, EBV Drugs – Heparin/Protamine HUS DIC Idiopathic Thrombocytopenic Purpura Autoimmune disease involving thrombocytopenia, purpura or petechiae, normal bone marrow function and no other known cause for decreased platelets. Autoantibodies attach to circulating platelets and are destroyed by the reticulendothelial system. Platelets function normally despite low numbers and bound with antibodies ITP – Acute and Chronic Course Acute – typically occurs in children, males = females, duration 1-2 months. Chronic – typically occurs in adults, lasts greater than 3 months, female > male, resolution unlikely even with treatment. Patients with chronic ITP more commonly have another disease or autoimmune disorder. ITP – PE and Lab Commonly PE will show petechiae, epistaxis, gingival bleeding, bruising, and possible menorrhagia. Remaining PE may be normal. Lab – CBC - low platelets, otherwise normal. Peripheral smear-normal platelets, few in number If history, PE, and above lab support the diagnosis of ITP, no further ED testing is necessary. Treatment - ITP Minimize bleeding risks – Meds, falls, comorbid disease states and procedures. Asymptomatic and healthy, with platelet counts > 50,000 – no treatment is needed < 50,000 and symptoms require treatment <20,000-30,000 require treatment Treatment - ITP Prednisone 60-100 mg/d – tapered after platelet count returns to normal ranges. If steroid therapy fails – splenectomy – produces remission in 65 percent of patients. If bleeding is life threatening – local hemorrhage control should instituted and high dose methylprednisolone 1-2 g/d for 2-3 days used. Intravenous immunoglobulin as needed. Platelets transfused after steroids or immunoglobins. Drug Induced Thrombocytopenia – Table 219-2 Common drugs Heparin Sulfas ASA Ethanol Thiazides Indomethacin Valproic Acid Lasix Platelet Sequestration Splenomegaly and thrombocytopenia without significant bleeding is not uncommon. Another bleeding disorder is usually involved if significant hemorrhage is present. Splenectomy is the definitive therapy for patients with low counts and evidence of significant hemorrhage. Qualitative Platelet Abnormalities Liver disease Myeloprolifertive Uremia disorders Dysproteinemias Von Willebrands disease Leukemias DIC SLE ITP Cardiopulmonary Bypass Drug Induced Platelet Dysfunction ASA NSAIDS Clopidogrel Ticlopidine Liver Disease Any disease that affects the hepatocytes can affect the production of the clotting factors Malabsorption of Vit. K by primary biliary cirrhosis and intra and extrahepatic cholestasis will affect factor II, VII, IV, and X In more severe liver disease, decreased synthesis of a2 plasmin inhibitor will cause a general state of fibrinolysis and increase DDimers and fibrin degradation products Lab To evaluate coagulation in liver disease the following labs will need to be ordered Hematocrit PT aPTT Platelet count FDP and D-Dimer Treatment Lab abnormalities without bleeding – patients can be observed Bleeding or invasive procedure needed – treatment is necessary Vitamin K for liver disease FFP if prolongation of PT and aPTT Cryopercipatate if fibrinogen levels < 100 mg/dL Platelet transfusion if indicated - <10,000 Renal Disease Dialysis related thrombocytopenia Toxin inhibition of platelet aggregation – uremic toxins. For life threatening bleeding, treatment is usually dialysis and transfusion for anemia, DDAVP and conjugated estrogens. Platelet transfusion and cryoprecipitate transfusions as needed. Disseminated Intravascular Coagulation DIC – an acquired syndrome characterized by activation of the coagulation system resulting in fibrin formation. The fibrinolytic system is also activated which breaks down clots, uses all coagulation proteins and results in bleeding - Tintinalli DIC is associated with many conditions – Table 219-5 Pathophysiology Disease process begins by the activation of tissue factor – extrinsic pathway. Thrombin converts fibrinogen to fibrin leading to formation of small clots that are deposited in capillaries causing tissue ischemia – organ dysfunction. Excessive activation of the coagulation system leads to depletion of coagulation proteins and platelets. tPA is activated indirectly by thrombin and fibrin and the fibrinolytic system is activated – in DIC the system works in excess and can result in uncontrolled bleeding DIC Manifestations Hemorrhage and thrombosis both take place, one form usually is dominate. Hemorrhage – most dominate Petechiae Ecchymoses GI/GU bleeding Wounds/IV sites DIC Manifestation Thrombosis Purpura fulminans Mental Status Changes MOF Oliguria ARDS Tissue necrosis In chronic DIC the liver produces enough coagulation proteins to compensate DIC - Lab Increased PT Thrombocytopenia – most common Low fibrinogen levels Fibrinogen levels less than 100 mg/dL, - more likely to see bleeding complications D-Dimers are more specific than FDPs in diagnosing DIC Increased LDH, decreased haptoglobin, schistocytes on peripheral smear DIC Treatment Treatment of underlying disease triggering DIC Hemodynamic support as needed –PRBCs, IVFs, vasopressors as needed. Supplementation of coagulation proteins, platelets and fibrinogen Cryoprecipitate in 10-unit doses to keep fibrinogen to 100-150 mg/dL Platelet transfusion if < 20,000 or < 50,000 with bleeding FFP transfused at 10-15 mL/kg to replace clotting proteins Vitamin K, Folate DIC Treatment Heparin for thrombotic dominant DIC or chronic DIC and known clots –purpura fulminans Antifibrinolytic agents are withheld for proven hypofibrinogenemia and fibrinolysis. Heparin should be infused before to decrease chance of thrombosis HIV Bleeding Disorders Thrombocytopenia is one of the earliest signs of HIV infection Bleeding problems are uncommon, the most common problems being bruising, mucosal bleeding and petechiae Thrombocytopenia caused by Increased destruction – immune complex related and HIV medications Decreased synthesis – immune complex related and HIV medications HIV Bleeding Disorders HIV patients commonly have Lupus anticoagulant – increased aPTT, which may appear and disappear with infection Anticardiolipin antibody – rarely causes a disorder in itself Circulating Anticoagulants Antibodies that affect coagulation factors Known inhibitors for each coagulation protein exist but the two most common are Factor VII inhibitors Antiphospholipid antibodies – lupus anticoagulant and anticardiolipin antibodies. Factor VIII Inhibitors Can develop at any time but usually affect patients with hemophilia A Patients at risk of developing factor VIII inhibitors Elderly Postpartum patients Autoimmune disorders – SLE, RA, UC, Mult. Myeloma Have drug reactions – PCNs, Sulfas, Phenytoin Factor VIII Inhibitors Signs and Symptoms Large bruising without trauma Ecchymoses Hematomas Lab Normal PT Normal Thrombin clotting time Prolonged aPTT – does not correct after mixing Factor VIII assay will be low Factor VIII Inhibitors Treatment of Bleeding Episodes Pressure to bleeding site Factor VIII concentrates Factor IX complex concentrates Prothrombin complex concentrates Recombinant factor VIIIa concentrates Plasmaphersis Ultimately a hematologist should direct care for life or limb threatening bleeding episodes Antiphospholipid Antibody Syndrome Presence of lupus anticoagulant or anticardiolipin antibodies, plus one or both of the following: thrombosis and/or complications with pregnancy (recurring fatal loss <34 weeks) Antiphospholipid Antibody Syndrome In reality SLE patients rarely have the antibody (5-15 percent) in vivo patients develop clots rather than bleed. Lupus anticoagulant more common in HIV patients, cancer, drug reactions and other autoimmune disorders Anticardiolipin antibodies commonly occur with lupus anticoagulant but both can occur separately Antiphospholipid Antibody Syndrome Lupus anticoagulant lab abnormalities Normal or prolonged PT Prolonged aPTT – does not correct when mixed Normal Thrombin clotting time Patients may develop antibodies to prothrombin causing a deficiency – suggested by markedly prolonged PT Factor assays – all factors will be mildly low Russell viper venom time – detects presence of lupus anticoagulant Anticardiolipin antibodies detected by ELISA assay Antiphospholipid Antibody Syndrome Signs and Symptoms Thromboembolism – venous > arterial Pregnancy complications Thrombocytopenia Patients who develop thrombosis and remain positive for lupus anticoagulant have a 50 percent chance of another clot forming within 2 years Recurrent fetal loss most likely due to thrombosis of placental vessels Antiphospholipid Antibody Syndrome Treatment Asymptomatic – observation, reduce risks for Virchows triad. Treat underlying disorder if known Corticosteroids for both AAS and autoimmune disease together Patients with episodes of thrombosis need lifelong anticoagulation ASA alone is inadequate LMWHs – good role for these medicines Bibliography Tintinalli Judith E., Emergency Medicine A Comprehensive Study Guide 6th Edition. Chapters 218-219 Questions T/F Lupus Anticoagulant is always present in patients with SLE? T/F von Willebrand disease is a disorder of the extrinsic clotting pathway? T/F Definitive treatment of DIC is heparin followed by coumadin therapy? T/F Defiencies of proteins C and S cause thrombotic disorders? T/F This was a fun and interesting chapter? F, F, F, T, F