Slide 1 - Elsevier
advertisement

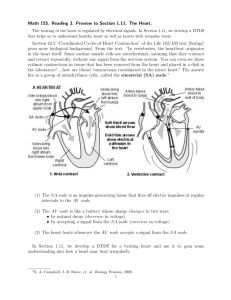
Chapter 7 Pathology of the Conduction System © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease FIGURE 7.1 Organization of the human cardiac conduction system. The sinoatrial node (SAN), generally positioned near the confluence of the superior caval vein (SCV) and the right atrium (RA), is the primary cardiac pacemaker. An electrical impulse generated by the SAN is conducted across the atria to the atrioventricular node (AVN), to the bundle of His (CB), and the left and right bundle branches (LBB, RBB). Finally, cardiac Purkinje fibers (PF) transmit the cardiac action potential throughout the myocardium. CS, coronary sinus; IVC, inferior vena cava; MB, moderator band; LV, RV, left and right ventricle; LA, RA, left and right atrium; PV, pulmonary vein; VS, ventricular septum. Reprinted from MRM Jongbloed, RV Steijn, ND Hahurij, TP Kelder, MJ Schalij, AC Gittenberger-de Groot and NA Blom. Normal and abnormal development of the cardiac conduction system; implications for conduction and rhythm disorders in the child and adult. Differentiation 2012;84:131–148, with permission from Elsevier. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 2 FIGURE 7.2 Organization of human sinoatrial node. Image of human right atrium depicting sinoatrial node arteries (dashed blue lines), sulcus terminalis (dashed white line), crista terminalis (CT), and the approximate border of the central sinoatrial node region (pink line). The black dashed line depicts location of the intra-atrial septum (IAS). (B) Transmural histological section from central sinoatrial node. Epicardium (epi), endocardium (endo). Reprinted from VV Fedorov, AV Glukhov, R Chang, G Kostecki, H Aferol, WJ Hucker, JP Wuskell, LM Loew, RB Schuessler, N Moazami, and IR Efimov. Optical mapping of the isolated coronary-perfused human sinus node. Journal of the American College of Cardiology 2010;56(17):1386–1394, with permission from Elsevier. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 3 FIGURE 7.3 Sinoatrial node cell structures. The morphology of isolated sinoatrial node cells varies widely from spindle (A) and elongated structures (B) to spider-type cell (C). For reference, an atrial cell is shown in (D). Reprinted from ME Mangoni and J Nargeot. Properties of the hyperpolarizationactivated current (I(f)) in isolated mouse sino-atrial cells. Cardiovascular Research 2001;52(1):51–64, with permission from Oxford University Press. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 4 FIGURE 7.4 Action potential morphologies of the cardiac conduction system. Left, image of the rabbit heart with predicted locations of the sinoatrial node, atrioventricular node, and His-Purkinje network. Right, action potential morphologies of different cardiac cell types. Note that different cardiac regions display differences in diastolic membrane potential (more positive in SAN vs. ventricle), action potential morphology, and duration. Reprinted from O Monfredi, H. Dobrzynski, T Mondal, MR Boyett, and GM Morris. The anatomy and physiology of the sinoatrial node – A contemporary review. Pacing and Clinical Electrophysiology, 2010;33(11):1392–1406, with permission from John Wiley and Sons. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 5 FIGURE 7.5 Structural view of the atrioventricular node. Organization of the human heart depicting the right atrium and the AV node (red). The image on the right represents a magnified image of the region of interest on the left. Ao, aorta; CN, compact node; CS, coronary sinus; FO, fossa ovalis; INE, inferior nodal extension; IVC, inferior vena cava; LBBB, left bundle branch block; PA, pulmonary artery; PB, penetrating bundle; RA, right atrium; RBBB, right bundle branch block; RV, right ventricle; SVC, superior vena cava; CFB, central fibrous body. Reprinted from IP Temple, S Inada, H Dobrinski, and MR Boyett. Connexins and the atrioventricular node. Heart Rhythm 2013;10:297–301, with permission from Elsevier. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 6 FIGURE 7.6 Organizational complexity of the cardiac His-Purkinje fiber system. Images of left and right ventricle (A,B) of mouse heart with transgenic expression enhanced green fluorescent protein under the control of the connexin40 gene promoter to illuminate the conduction system. The dashed line in (A) denotes the limits between the left flank (LF) of the IVS and the left ventricular wall (LVW). In (B), the dashed white line denotes the border between the right ventricular wall (RVW) and the right flank (RF) of the IVS. RVW, right ventricular wall; PF, Purkinje fibers; LBB, RBB, left and right bundle branch; HB, His bundle; APM, anterior papillary muscle; circles denote connecting fibers and asterisk denotes the septal artery. Reprinted from L Miquerol, S Meysen, M Mangoni, P Bois, HVM van Rijen, P Abran, H Jongsma, J Nargeot, and D Gros. Architectural and functional asymmetry of the His-Purkinje system of the murine heart. Cardiovascular Research, 2004;63:77-86, with permission from Oxford University Press. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 7 FIGURE 7.7 Specialized morphology of cardiac conduction system Purkinje fibers. (A,B) Rat Purkinje fibers (arrowheads) with surrounding layer of collagen. (C) Cross-section of Purkinje fiber. Asterisks show collagen where arrows denote glycogen. (D,E) Comparison of Purkinje fiber (D) and ventricular myocyte (E) intercalated disc junction. Arrows denote intercalated disc region. Note the elongated border between adjacent Purkinje cells. Reprinted from W Dun and PA Boyden. The Purkinje cell. Heart Rhythm 2008;45:617–624, with permission from Elsevier. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 8 FIGURE 7.8 Ankyrin-B dysfunction results in human conduction disease and arrhythmia. Humans and mice harboring ankyrin-B (ANK2) loss of function mutations display bradycardia and heart rate variability at rest. Moreover, humans and mice with ankyrin-B loss of function mutations display increased susceptibility to severe ventricular arrhythmia and susceptibility to death following elevated catecholamine levels. Top panel depicts wildtype mouse, lower panel depicts ankyrin-B heterozygous mouse. Reprinted from S DeGrande, D Nixon, O Koval, JW Curran, P Wright, Q Wang, F Kashef, D Chiang, N Li, XHT Wehrens, ME Anderson, TJ Hund, and PJ Mohler. CaMKII inhibition rescues proarrhythmic phenotypes in the model of human ankyrin-B syndrome. Heart Rhythm 2012;9:2034–2041, with permission from Elsevier. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 9