Solvent Effects
advertisement
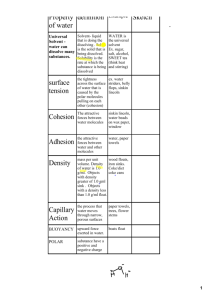
Organic Pedagogical Electronic Network Solvent Effects Laura Beuth, Jenni Frosch & Hilke Wiedenroth Solvent Effects Overview: Different solvents can have different effects on the same reaction by influencing the solubility and stability of reactants and transition states, thereby also influencing reaction rates. Based on solvent selection, the reaction pathways leading to thermodynamic versus kinetic product profiles may be controlled. Solubility: Solutes (molecules to dissolve) dissolve when they experience non-covalent intermolecular interactions with a solvent that are similar to those interactions experienced between solute molecules. Molecules that are polar will dissolve in polar solvents; molecules are non-polar will dissolve in non-polar solvents. Reaction equilibria will lie in the direction favoring formation of the better solubilized species. Stability: Better solubility corresponds to greater stability, and reaction equilibria adjust to favor formation of the more stabilized species. For example, in 1,3-dicarbonyl compounds, two tautomers exist in concentrations that vary according to solvent. The diketo form is better stabilized by polar solvents; the enol form is better stabilized by non-polar solvents. The more stabilized form exists in a greater concentration relative to the less stabilized form. This is evidenced by measuring and comparing equilibrium constants of tautomerization (KT) in solvents of varying polarity. Solvent KT Cyclohexane 1.65 Acetone 0.13 Water 0.07 KT = [enol]/[keto] Keto Enol Reaction rates: Solvents can influence reaction rates by (de)stabilizing transition states. For instance, charged species will be stabilized by polar solvents. If charge builds up in a molecule over the course of a reaction, then a polar (relative to a non-polar) solvent can increase the reaction rate by stabilizing this charge and reducing the energetic barrier to its formation. Conversely, if greater charge exists in the starting material than in later reaction intermediates, a polar solvent will stabilize the starting material, impeding the reaction’s progress. Wiki Page: http://en.wikipedia.org/wiki/Solvent_effects Other References: Reichardt, C.; Welton, T., Solvents and Solvent Effects in Organic Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA: 2010. Solvent-Controlled Product Formation Solvent Effects on Lithiation: In the polar solvent diethyl ether, molecule 1 is first lithiated, which involves a deprotonation of a vinyl proton to yield the isolated species 2. An SN2 reaction ensues as the trimethylsilyl chloride undergoes nucleophilic attack by the lithiated carbon to afford 3. Conversely, in the less polar solvent mixture hexane/diethyl ether (1:1 v/v), tBuLi adds across the P=C bond, giving ia and ib, which directly react to form 5 and 4, respectively. The different products afforded in these reactions, under polar versus less polar solvent conditions, can be attributed to the ability of the solvent to differently solvate the tBuLi reagent. The polarity of diethyl ether enables stabilization, and, thus, solvation, of each ion in the lithiate pair. Solvation of each ion results in tBuLi reacting as a base in a C–H lithiation manner to yield 2. The less polar solvent mixture (hexane:diethyl ether) is less able to solvate each ion in the lithiate. Therefore, the reagent reacts as a nucleophile in its addition across the P=C bond. References: Ghalib, M.; Jones, P. G.; Heinicke, J. W. Solvent-Controlled Lithiation of Pc–N-Heterocycles: Synthesis of Mono- and Bis(Trimethylsilyl)-TertButyl-Dihydrobenzazaphospholes – a New Type of Highly Bulky And basic phosphine Ligands. J. Organomet. Chem. 2014, 763–764, 44-51. Solvent Effects on Reaction Rate 2 [FeIIL3]2+ + S2O82- → 2 [FeIIIL3]3+ + 2 SO42L = bpai, bpmai, bpfai Solvent Effects on Reaction Rates: The observed rate constant (kobs) of the above iron oxidation reaction varies according to the solvent in which the reaction is performed. Solvents that better stabilize the starting FeII complex will yield lower reaction rates, and solvents that better stabilize the transition state will result in increased reaction rates. Determining how polar and non-polar solvents may differentially impact rates provides information about the hydrophilic/hydrophobic nature of the starting material and transition state. The table below presents the impact on reaction rates of changing the percentage of methanol co-solvent that is added to water. More polar mixtures (i.e., those with lower percentages of methanol) afford greater reaction rates. This implies that the transition state is more stabilized by more polar solvents, resulting in a lower activation energy to reach the transition state. A transition state that is better stabilized by more polar solvents indicates that the transition state is more hydrophilic than the starting complex. Even small amounts of the methanol organic co-solvent increases the freeenergy barrier to the transition state and, thus, decrease the reaction rate. This means that the starting FeII complex is more hydrophobic than the transition states. 105 kobs (s-1) Percent Methanol in Water Ligand 0 10 20 30 bpai 2.74 2.10 1.67 1.27 bpmai 4.26 3.29 2.69 1.96 bpfai 4.65 3.22 2.82 2.44 References: Ebaid Nassr, L. A.-M.; Shaker, A. M. Kinetic and Mechanistic Aspects of Reactivity of the Oxidation of Some Fe(Ii)–Tris Schiff Base Complexes by Peroxydisulfate: Spectrophotometric Tracer and Solvent Effect on the Reactivity. Int. J. Chem. Kinet. 2014, 46, 701-710. Problems 1. What can you infer about the relative polarities of the solvents in the below reaction schemes based on the given product distributions? 2. Will a polar or non-polar solvent give the greatest reaction rates for the below reaction? Why? Solution 1. Product 2 is the result of a C–H lithiation reaction, and 3 may result from the reaction of 2 through a nucleophilic addition of tBuLi across the P=C bond. Lithiations require solvation of tBuLi such that the lithium-alkyl interaction is more ionic than covalent. An ionic interaction is allowed when polar solvents can stabilize the ions independent of an oppositely charged ion. This enables tBu to behave as a base, resulting in a C–H lithiation reaction. Thus, the hexane/THF mixture is sufficiently polar to enable lithiation. Alternatively, in toluene solvent, products 4 and 5 result from tBuLi behaving as a nucleophile. When lithiates are in a less polar environment and cannot be stabilized as an ion pair, this species reacts as a nucleophile. These results indicate that the hexane/THF solvent mixture is more polar then toluene. 2. This is an SN2 reaction, so a less polar solvent will favor a faster rate of reaction. The starting material is more polar than the transition state, so a solvent matching the polarity of the starting material would stabilize the starting material and reduce the reactivity of the substrate. References: Ghalib, M.; Jones, P. G.; Heinicke, J. W. Solvent-Controlled Lithiation of Pc–N-Heterocycles: Synthesis of Mono- and Bis(Trimethylsilyl)-TertButyl-Dihydrobenzazaphospholes – a New Type of Highly Bulky And basic phosphine Ligands. J. Organomet. Chem. 2014, 763–764, 44-51.