Slides/Metabolism 2013 Thurs pt 1
advertisement
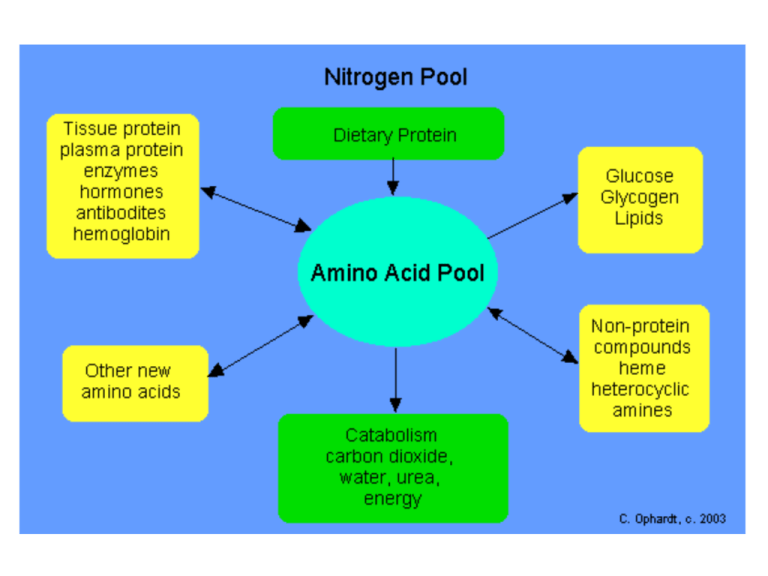
Amino Acid Metabolism All tissues in the body have limited capability for synthesis of: •Non-essential or dispensable amino acids •Amino acid remodeling •Conversion of non-amino acid carbon skeletons into amino acids •Conversion of non-animo acid carbon skeletons into derivatives that contain nitrogen Amino Acid Metabolism The liver is the major site of nitrogen metabolism •In times of dietary surplus, the potentially toxic nitrogen of amino acids is eliminated via transaminations, oxidative deamination, and urea formation. Carbon skeletons are generally conserved: •carbohydrate, via gluconeogenesis •fatty acid via fatty acid synthesis •During times of starvation the carbon skeletons of amino acids are reduced and used for energy production (oxidized to CO2 and H2O). Amino Acid Metabolism Amino acids fall into three categories: 1. Glucogenic: which give rise to a “net” production of pyruvate or TCA cycle intermediates, such as α-ketoglutarate or oxaloacetate (precursors to glucose via gluconeogenesis) All amino acids except Lysine and Leucine are at least partly glucogenic. 2. Ketogenic: which give rise to acetylCoA or acetoacetylCoA Lysine and Leucine are solely ketogenic 3. Glucogenic and Ketogenic: which give rise to both glucose and fatty acid precursors Isoleucine, Phenylalanine, Threonine, Tryptophan, and Tyrosine Non-essential Amino Acid Biosynthesis Glutamate Glutamate is synthesized from the widely distributed α-keto acid precursor by a simple 1step transamination reaction catalyzed by glutamate dehydrogenase Non-essential Amino Acid Biosynthesis Aspartate Aspartate is synthesized by a simple 1-step transamination reaction catalyzed by aspartate aminotransferase, AST (formerly referred to as serum glutamate-oxalate transaminase, SGOT). Non-essential Amino Acid Biosynthesis Aspartate Aspartate can also be derived from asparagine through the action of asparaginase. Aspartate is also a precursor of ornithine (urea cycle). Non-essential Amino Acid Biosynthesis Alanine There are 2 main pathways for production of alanine: directly from protein degradation or transamination of pyruvate by alanine transaminase, ALT (also referred to as serum glutamate-pyruvate transaminase, SGPT). Non-essential Amino Acid Biosynthesis Alanine Alanine is second only to glutamine in circulating amino acid abundance. Alanine is transferred to circulation mainly by muscle Alanine is formed from pyruvate at a rate proportional to intracellular pyruvate levels Liver accumulates plasma alanine, reverses the transamination that occurs in muscle, and proportionately increases urea production Pyruvate is either oxidized or converted to glucose When alanine transfer from muscle to liver is coupled with glucose transport from liver back to muscle, the process is known as the glucose-alanine cycle. This serves to essentially transfer nitrogen from peripheral tissue to the liver. Muscle gets rid of nitrogen and replenishes its energy supply Non-essential Amino Acid Biosynthesis Cysteine The sulfur for cysteine synthesis comes from the essential amino acid methionine. A condensation of ATP and methionine catalyzed by methionine adenosyltransferase yields S-adenosylmethionine (SAM or AdoMet). Non-essential Amino Acid Biosynthesis Cysteine SAM serves as a precurosor for numerous methyl transfer reactions (e.g. the conversion of norepinephrine to epinenephrine). Methyl Transfer The result of methyl transfer is the conversion of SAM to S-adenosylhomocysteine which is then cleaved by adenosylhomocyteinase to yield homocysteine and adenosine. Trans-sulfuration Homocysteine condenses with serine to produce cystathionine, which is cleaved by cystathionase to produce cysteine and α-ketobutyrate. Cysteine is used for protein synthesis α-ketobutyrate is first converted to propionyl-CoA then to succinyl-CoA. Cysteine readily oxidizes in air to form the disulfide cystine but, cells contain little or no free cystine Non-essential Amino Acid Biosynthesis Tyrosine Tyrosine is produced in cells by hydroxylating the essential amino acid phenylalanine. Half of the phenylalanine required goes into the production of tyrosine; if the diet is rich in tyrosine itself, the requirements for phenylalanine are reduced by about 50%. PKU in Humans Missing or deficient phenylalanine hydroxylase results in hyperphenylalaninemia. Hyperphenylalaninemia is defined as a plasma phenylalanine concentration greater than 2mg/dL (120μM). The most widely recognized hyperphenylalaninemia (and most severe) is the genetic disease known as phenylketonuria or PKU. Patients suffering from PKU have plasma phenylalanine levels >1000μM, whereas the non-PKU humans exhibit levels of plasma phenylalanine <1000μM. Untreated PKU leads to severe mental retardation which is caused by the accumulation of phenylalanine and depletes neural tissue of α-ketoglutarate. This absence of α-ketoglutarate in the brain shuts down the TCA cycle. Non-essential Amino Acid Biosynthesis Proline Glutamate is the precursor of both proline and ornithine. Glutamate semialdehyde is the branch point intermediate leading to one of these 2 products. The production of ornithine from glutamate is important when dietary arginine (the other principal source of ornithine) is limited. Non-essential Amino Acid Biosynthesis Serine The main pathway to de novo biosynthesis of serine starts with the glycolytic intermediate 3phosphoglycerate. An NADH-linked dehydrogenase converts 3-phosphoglycerate into a keto acid, 3-phosphopyruvate, suitable for subsequent transamination. Aminotransferase activity with glutamate as a donor produces 3-phosphoserine, which is converted to serine by phosphoserine phosphatase. Non-essential Amino Acid Biosynthesis Glycine The main pathway to glycine is a 1-step reversible reaction catalyzed by serine hydroxymethyltransferase (SHMT). This reaction involves the transfer of the hydroxymethyl group from serine to the cofactor tetrahydrofolate (THF), producing glycine and N5,N10methylene-THF. There are mitochondrial and cytosolic versions of serine hydroxymethyltransferase. The cytosolic enzyme is SHMT1 The mitochondrial enzyme is SHMT2. Glycine is involved in many anabolic reactions other than protein synthesis including the synthesis of purine nucleotides, heme, glutathione, creatine and serine. Glycine produced from serine or from the diet can also be oxidized by glycine decarboxylase (also referred to as the glycine cleavage complex, GCC) to yield a second equivalent of N5,N10-methylene-tetrahydrofolate as well as ammonia and CO2. Non-essential Amino Acid Biosynthesis Aspartate/Asparagine and Glutamate/Glutamine Biosynthesis Glutamate is synthesized by the reductive amination of α-ketoglutarate catalyzed by glutamate dehydrogenase; it is thus a nitrogen-fixing reaction. In addition, glutamate arises by aminotransferase reactions, with the amino nitrogen being donated by a number of different amino acids. Thus, glutamate is a general collector of amino nitrogen. Non-essential Amino Acid Biosynthesis Asparagine synthetase and glutamine synthetase, catalyze the production of asparagine and glutamine from their respective α-amino acids. Glutamine is produced from glutamate by the direct incorporation of ammonia; and this can be considered another nitrogen fixing reaction. Asparagine, however, is formed by an amidotransferase reaction. Aminotransferase reactions are readily reversible. The direction of any individual transamination depends principally on the concentration ratio of reactants and products. Transamidation reactions, which are dependent on ATP, are considered irreversible. Thus, degradation of asparagine and glutamine take place by a hydrolytic pathway rather than by a reversal of the pathway by which they were formed. Amino Acid Catabolism Glutamine/Glutamate and Asparagine/Aspartate Catabolism Glutaminase is an important kidney tubule enzyme involved in converting glutamine (from liver and from other tissue) to glutamate and NH4+, with the NH4+ being excreted in the urine. Glutaminase activity is present in many other tissues as well, although its activity is not nearly as prominent as in the kidney. The glutamate produced from glutamine is converted to αketoglutarate, making glutamine a glucogenic amino acid. Glutamine/Glutamate and Asparagine/Aspartate Catabolism Asparaginase is also widely distributed within the body, where it converts asparagine into ammonia and aspartate. Aspartate transaminates to oxaloacetate, which follows the gluconeogenic pathway to glucose. Glutamate and aspartate are important in collecting and eliminating amino nitrogen via glutamine synthetase and the urea cycle, respectively. The catabolic path of the carbon skeletons involves simple 1-step aminotransferase reactions that directly produce net quantities of a TCA cycle intermediate. The glutamate dehydrogenase reaction operating in the direction of α-ketoglutarate production provides a second avenue leading from glutamate to gluconeogenesis. Alanine Catabolism Alanine is also important in intertissue nitrogen transport as part of the glucose-alanine cycle. Alanine's catabolic pathway involves a simple aminotransferase reaction that directly produces pyruvate. Generally pyruvate produced by this pathway will result in the formation of oxaloacetate, although when the energy charge of a cell is low the pyruvate will be oxidized to CO2 and H2O via the PDH complex and the TCA cycle. This makes alanine a glucogenic amino acid. Arginine, Ornithine and Proline Catabolism The catabolism of arginine begins within the context of the urea cycle. It is hydrolyzed to urea and ornithine by arginase. Ornithine, in excess of urea cycle needs, is transaminated to form glutamate semialdehyde. Glutamate semialdehyde can serve as the precursor for proline biosynthesis or it can be converted to glutamate. Proline catabolism is a reversal of its synthesis process. The glutamate semialdehyde generated from ornithine and proline catabolism is oxidized to glutamate by an ATP-independent glutamate semialdehyde dehydrogenase. The glutamate can then be converted to α-ketoglutarate in a transamination reaction. Thus, arginine, ornithine and proline, are glucogenic. Serine Catabolism The conversion of serine to glycine and then glycine oxidation to CO2 and NH3, with the production of two equivalents of N5,N10-methyleneTHF, (glycine biosynthesis). Serine can be catabolized back to the glycolytic intermediate, 3-phosphoglycerate, by a pathway that is essentially a reversal of serine biosynthesis. It has been demonstrated in rodents and dogs that serine can be converted to pyruvate through a deamination reaction catalyzed by serine/threonine dehydratase. Glycine Catabolism Glycine is classified as a glucogenic amino acid, since it can be converted to serine by serine hydroxymethyltransferase and serine can be converted back to the glycolytic intermediate, 3-phosphoglycerate . Serine can also be converted to pyruvate by serine/threonine dehydratase. This does not occur in humans. The main glycine catabolic pathway leads to the production of CO2, ammonia, and one equivalent of N5,N10-methyleneTHF by the mitochondrial glycine decarboxylase, also called the glycine cleavage complex, GCC. Threonine Catabolism The principal threonine catabololizing pathway in humans involves glycine-independent serine/threonine dehydratase yielding α-ketobutyrate which is further catabolized to propionylCoA and finally to succinyl-CoA. Serine/threonine dehydratase is expressed at high levels only in the liver. It appears that in newborn infants catabolism of threonine occurs exclusively via the action of the serine/threonine dehydratase. Therefore, it is presumed that this is the predominant threonine catabolizing pathway in humans. The second pathway of threonine catabolism utilizes serine hydroxymethyltransferase. The products of this reaction are acetyl-CoA and glycine. The glycine can be converted to serine via the same enzyme and the serine is then catabolized yielding pyruvate and NH4+. Thus, via this catabolic pathway threonine yields ketogenic and glucogenic byproducts. In humans it appears that threonine aldolase is actually encoded by a non-functional pseudogene, whereas in other vertebrates the threonine aldolase gene encodes a functional threonine catabolizing enzyme. An additional pathway occurs in the mitochondria and is initiated by threonine dehydrogenase eventually yielding to acetyl-CoA and glycine. The threonine dehydrogenase gene in humans appears to be non-functional due to the incorporation of three inactivating mutations. Cysteine Catabolism There are several pathways for cysteine catabolism. The simplest, but least important pathway is catalyzed by a liver desulfurase and produces hydrogen sulfide, (H2S) and pyruvate. The major catabolic pathway in animals is via cysteine dioxygenase that oxidizes the cysteine sulfhydryl to sulfinate, producing the intermediate cysteinesulfinate. Cysteinesulfinate can serve as a biosynthetic intermediate undergoing decarboxylation and oxidation to produce taurine. Catabolism of cysteinesulfinate proceeds through transamination and eventualy leads to pyruvate. Other than protein, the most important product of cysteine metabolism is the bile salt precursor taurine, which is used to form the bile acid conjugates taurocholate and taurochenodeoxycholate. Methionine Catabolism Methionine is: Incorporated into polypeptide chains Used in the production of α-ketobutyrate Used in the production of cysteine via SAM The transulfuration reactions that produce cysteine from homocysteine and serine also produce α-ketobutyrate, the latter being converted first to propionyl-CoA and then via a 3-step process to succinyl-CoA. Valine, Leucine and Isoleucine Catabolism This group of essential amino acids are identified as the branched-chain amino acids, BCAAs. Because this arrangement of carbon atoms cannot be made by humans, these amino acids are an essential element in the diet. The catabolism of all three compounds initiates in muscle and yields NADH and FADH2 which can be utilized for ATP generation. The catabolism of all three of these amino acids uses the same enzymes in the first two steps. The first step in each case is a transamination using a single BCAA aminotransferase, with αketoglutarate as amine acceptor. As a result, three different α-keto acids are produced and are oxidized using a common branched-chain α-keto acid dehydrogenase (BCKD), yielding the three different CoA derivatives. Valine breakdown produces propionylCoA, the glucogenic precursor of succinyl-CoA. Isoleucine catabolism terminates with production of acetylCoA and propionylCoA; thus isoleucine is both glucogenic and ketogenic. Leucine gives rise to acetylCoA and acetoacetylCoA, and is thus classified as strictly ketogenic. Phenylalanine and Tyrosine Catabolism Phenylalanine has only two fates: incorporation into polypeptide chains production of tyrosine Phenylalanine catabolism always follows the pathway of tyrosine catabolism. The main pathway for tyrosine degradation involves conversion to fumarate and acetoacetate, allowing phenylalanine and tyrosine to be classified as both glucogenic and ketogenic. Tyrosine is equally important for protein biosynthesis as well as an intermediate in the biosynthesis of several physiologically important metabolites e.g. dopamine, norepinephrine and epinephrine. Lysine Catabolism Lysine catabolism is unusual in the way that the ε-amino group is transferred to α-ketoglutarate and into the general nitrogen pool. The reaction is a transamination in which the ε-amino group is transferred to the α-keto carbon of α-ketoglutarate forming the metabolite, saccharopine. Unlike the majority of transamination reactions, this one does not employ pyridoxal phosphate as a cofactor. Because this transamination reaction is not reversible, lysine is an essential amino acid. The ultimate end-product of lysine catabolism is acetoacetyl-CoA. Lysine is also important as a precursor for the synthesis of carnitine, required for the transport of fatty acids into the mitochondria for oxidation. Free lysine does not serve as the precursor for this reaction, rather the modified lysine found in certain proteins. Some proteins modify lysine to trimethyllysine using SAM as the methyl donor to transfer methyl groups to the ε-amino of the lysine side chain. Hydrolysis of proteins containing trimethyllysine provides the substrate for the subsequent conversion to carnitine. Histidine Catabolism Histidine catabolism begins with release of the α-amino group catalyzed by histidase, introducing a double bond into the molecule. The end product of histidine catabolism is glutamate, making histidine one of the glucogenic amino acids. Another key feature of histidine catabolism is that it serves as a source of ring nitrogen to combine with tetrahydrofolate (THF). Tryptophan Catabolism A number of important side reactions occur during the catabolism of tryptophan on the pathway to acetoacetate. Kynurenine is the first key branch point intermediate in the catabolic pathway leading to 3 fates: Kynurenine can undergo deamination in a standard transamination reaction yielding kynurenic acid. High levels of kynurenic acid have been found in the urine of individuals suffering from schizophrenia. Aside from its role as an amino acid in protein biosynthesis, tryptophan also serves as a precursor for the synthesis of serotonin and melatonin. The Urea Cycle Kidney glutaminase is responsible for converting excess glutamine from the liver to urine ammonium. However, about 80% of the excreted nitrogen is in the form of urea which is also largely made in the liver, in a series of reactions that are distributed between the mitochondrial matrix and the cytosol. The series of reactions that form urea is known as the Urea Cycle or the Krebs-Henseleit Cycle. CPS-I is carbamoyl phosphate synthetase-I, OTC is ornithine transcarbamoylase. Regulation of the Urea Cycle The urea cycle operates only to eliminate excess nitrogen. On high-protein diets the carbon skeletons of the amino acids are oxidized for energy or stored as fat and glycogen, but the amino nitrogen must be excreted. Enzymes of the urea cycle are controlled at the gene level. With long-term changes in the quantity of dietary protein, changes of 20-fold or greater in the concentration of cycle enzymes are observed. When dietary proteins increase significantly, enzyme concentrations rise. On return to a balanced diet, enzyme levels decline. Under conditions of starvation, enzyme levels rise as proteins are degraded and amino acid carbon skeletons are used to provide energy, thus increasing the quantity of nitrogen that must be excreted. Regulation of the Urea Cycle Short-term regulation of the cycle occurs principally at CPS-I, which is relatively inactive in the absence of its allosteric activator N-acetylglutamate. The steady-state concentration of N-acetylglutamate is set by the concentration of its components acetyl-CoA and glutamate and by arginine, which is a positive allosteric effector of N-acetylglutamate synthetase.




