Photobiology
advertisement

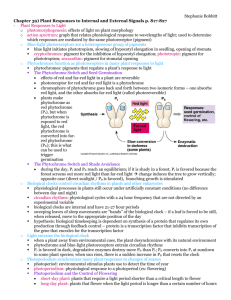
Photobiology 3rd Year Student of biophysics Prepared By Prof. Dr. Mohammed Naguib Abd El-Ghany Hasaneen Professor Of Plant Metabolism And Biotechnology Academic Year 2005 - 2006 Contents • • • • • • • • Introduction Radiation Visible light Ultraviolet light Ultraviolet light damage Phytochrome concept Distribution and translocation of phytochrome Physiological effects of phytochrome Introduction Light in Plants We see visible light (350-700 nm) Plants sense Ultra violet (280) to Infrared (800) Examples Seed germination - inhibited by light Stem elongation- inhibited by light Shade avoidance- mediated by far-red light There are probably 4 photoreceptors in plants We will deal with the best understood; PHYTOCHROMES A Primer on Radiation Some important plant responses to radiation (“light” is only one form of radiation): •Photosynthesis •Photomorphogenesis; • Photropism; Photoperiodism •Energy balance/temperature •respiration •enzyme activity •transpiration •UV-responses •mutagenesis (note that there is a much more detailed table and discussion of responses of plants to light in chapter 1 of Hart: Light and Plant Growth) In what form does energy from the sun travel to Earth? • • • Energy travels to Earth in the form of electromagnetic waves Electromagnetic waves are classified according to wave length Radiation is the direct transfer of energy by electromagnetic waves Most of the energy from the sun reaches Earth in the form of • Visible light • Infrared radiation • A small amount of ultraviolet radiation • The different colors of light make up the visible spectrum. • Red has the longest wave length • Violet has the shortest wave length Infrared radiation has the following properties: • • • • Wavelengths longer than red light It is not visible It can be felt as heat Used to warm food or baby chicks in an incubator Ultraviolet light has the following properties: • Wave lengths shorter than violet light • Can cause skin damage • Can cause eye problems Radiation and radiation laws The way we describe and quantify radiation, and the units used, vary depending on the kind of process we’re interested in Properties of radiation that are important to plants include Quality, Quantity, Direction (including diffuse vs. direct) and Periodicity. Radiation quality (or “color”, for visible light) is a function of its wavelength (or frequency) distribution Note these two charts are arrayed in opposite directions – one by increasing wavelength/decreasing energy and the other by increasing frequency/increasing energy The symbol “l” is often used for wavelength Radiation measurements Radiation quantity is measured in one of three ways, depending on the application: 1. Quantum measurements (numbers of photons) 2. Radiometric measurements (amount of energy) 3. Photometric measurements (light intensity, based on human perception) The amount of radiation is expressed as fluence (also known as density; quantity per area), rate (also known as flux; quantity per time) or fluence rate (also known as flux density; amount per area per time) Parameter Term Energy units Quantum units Quantity per area fluence J m-2 mmol m-2 Quantity per time rate (or flux) J s-1 (watt) mmol s-1 Quantity per area per time fluence rate (or W m-2 flux density) mmol m-2 s-1 For studies of photosynthesis and photomorphogenesis, the quantity of radiation is usually measured in quantum units (quantum flux density; quantum fluence rate): mmol m-2 s-1 Note that “mol” refers to a mole of photons, and that 1 mol photons=1 Einstein. A quantum is one indivisible “package” of radiation, or one photon. usually, only the visible, or photosynthetically active part of the spectrum is measured, or in the case of photomorphogenesis, only specific wavelengths PPFD = photosynthetically active photon flux density PAR = photosynthetically active radiation (400-700 nm) For energy balance studies, radiation is measured in radiometric units, for example: Watts m-2 (note: 1 Watt = 1 Joule s-1) Radiometric and quantum units are interconverted based on the amount of energy in photons. The energy of a photon is proportional to its frequency and inversely proportional to wavelength: Energy per photon (joules) Planck’s constant: 6.63 x 10-34 joules s E = hn = hc/l wavelength (in meters) Frequency (s-1) Speed of light 3 X 108 m s-1 See link from website to “working with light” or p. 28 of the handout by Hart or any good reference on radiation for more information on this conversion) Because most light sources contain a wide range of wavelengths, it is difficult to convert precisely between quantum and radiometric units. Usually an approximation is used that assumes a “typical” distribution of wavelengths for a particular light source All objects emit radiation (i.e., they “radiate”) as a function of their temperature (in addition to the emissivity of the material). Temperature affects both the amount and the quality (wavelength) of radiation emitted. Wien’s Law: l max = 2897/T Temperature of radiating body, in degrees Kelvin The “bulk” of solar radiation is “shortwave” (visible plus near infrared) Notice that the range of photosynthetically active wavelengths is very small relative to the range of the solar spectrum Spectral Quality • visible = 400-700 nm, about 45% of incident insolation • solar IR = 700-5000 nm, about 46% of incident • UV = 190-400 nm, about 9% of incident • Restating this as a rough “rule of thumb”: When the sky is clear, the photosynthetically active part of the solar spectrum accounts for about HALF of the total solar energy, IR accounts for the other half Radiance vs. Irradiance: Radiance is the radiation that is emitted from an object In this case, radiation is commonly described as a flux (rate), or amount per unit time. This could be either a radiant flux or a quantum flux Irradiance is the radiation that impinges upon an object In this case, radiation is commonly described as a flux density, or amount per unit time per unit area. Again, the flux could be quantified either with either radiometric or photometric units. Irradiance usually has both direct and diffuse components: Direct irradiance Diffuse irradiance The amount of energy in direct-beam irradiance is strongly affected by the angle between the surface and the beam Lambert’s Cosine Law: Solar angle and leaf angle can have a very big influence on irradiation, dramatically affecting photosynthesis, transpiration and leaf temperature definitions: Heliotropic: “sun tracking” Paraheliotropic: leaf stays parallel to direct beam of sun Diaheliotropic: leaf stays perpendicular to direct beam Connections between matter and energy A short, painless review of simple organic chemistry …… to develop the connection between cycles of organic biomass and cycles of energy (Inorganic; not a hydrocarbon. This is a highly oxidized form of carbon) methane ethane ethene ethyne (organic hydrocarbons. The molecules are becoming increasingly reduced) increasing potential energy (energy stored in chemical bonds) CO2 general deterioration of #4 green shade from trees and tower general deterioration of #4 green shade from trees and tower poor air circulation from trees and shrubs general deterioration of #4 green concentrated traffic between trap and green shade from trees and tower poor air circulation from trees and shrubs general deterioration of #4 green concentrated traffic between trap and green poor internal and surface drainage shade from trees and tower poor air circulation from trees and shrubs general deterioration of #4 green concentrated traffic between trap and green poor internal and surface drainage shade from trees and tower poor air circulation from trees and shrubs delicate turfgrass hot, humid microenvironment general deterioration of #4 green O2-deficient rootzone concentrated traffic between trap and green poor internal and surface drainage Wavelength - ENERGY • Photons in short wavelengths pack a lot of energy – Visible light (400-750nm): • 1 mole of photons = 250kJ energy – Ultraviolet light (< 400 nm): • 1 mole of photons = 500 kJ energy • Photons in longer wavelengths do not – Infrared radiation (>750 nm) • 1 mole of photons = 85 kJ energy • What happens when sunlight hits the wall of a building? – Some reflected back to space (no effect) (this depends upon the COLOR of the wall!) – Most is absorbed. Then what? • Absorption of radiation makes the temperature of the object rise • How hot? • The hotter the more radiation emitted (as infrared) • Heats until energy in = energy out • Or energy absorbed = energy re-radiated The Thermal Environment • Energy is gained and lost through various pathways: – radiation - all objects emit electromagnetic radiation and receive this from sunlight and from other objects in the environment – conduction - direct transfer of kinetic energy of heat to/from objects in direct contact with one another – convection - direct transfer of kinetic energy of heat to/from moving air and water – evaporation - heat loss as water is evaporated from organism’s surface (2.43 kJ/g at 30oC) change in heat content = metabolism - evaporation + radiation + conduction + convection Organisms must cope with temperature extremes. • Unlike birds and mammals, most organisms do not regulate their body temperatures. • All organisms, regardless of ability to thermoregulate, are subject to thermal constraints: – most life processes occur within the temperature range of liquid water, 0o-100oC – few living things survive temperatures in excess of 45oC – freezing is generally harmful to cells and tissues So how do organisms regulate temperature? • Manipulating the energy balance equation! – Net radiation • Color, Orientation to sun, Minimizing/maximize IR losses (insulation) – Conduction • Use warm or cool surfaces – Convection: • Minimize or maximize exposure to wind or water (boundary layers, exposure, immersion) – Evaporation: • Minimize or maximize evaporation to control heat loss – Metabolism: Generate or limit generation of heat! • These can be morphological, physiological, or behavioral adaptations Conserving Water in Hot Environments • Animals of deserts may experience environmental temperatures in excess of body temperature: – evaporative cooling is an option, but water is scarce – animals may also avoid high temperatures by: • reducing activity • seeking cool microclimates • migrating seasonally to cooler climates Conserving Water in Hot Environments • Desert plants reduce heat loading in several ways already discussed. Plants may, in addition: – orient leaves to minimize solar gain – shed leaves and become inactive during stressful periods The Kangaroo Rat - a Desert Specialist • These small desert rodents perform well in a nearly waterless and extremely hot setting. – kangaroo rats conserve water by: • producing concentrated urine • producing nearly dry feces • minimizing evaporative losses from lungs – kangaroo rats avoid desert heat by: • venturing above ground only at night • remaining in cool, humid burrow by day Tolerance of Freezing • Freezing disrupts life processes and ice crystals can damage delicate cell structures. • Adaptations among organisms vary: – maintain internal temperature well above freezing – activate mechanisms that resist freezing • glycerol or glycoproteins lower freezing point effectively (the “antifreeze” solution) • glycoproteins can also impede the development of ice crystals, permitting “supercooling” – activate mechanisms that tolerate freezing Organisms maintain a constant internal environment. • An organism’s ability to maintain constant internal conditions in the face of a varying environment is called homeostasis: – homeostatic systems consist of sensors, effectors, and a condition maintained constant – all homeostatic systems employ negative feedback -- when the system deviates from set point, various responses are activated to return system to set point Temperature Regulation: an Example of Homeostasis • Principal classes of regulation: – homeotherms (warm-blooded animals) maintain relatively constant internal temperatures – poikilotherms (cold-blooded animals) - tend to conform to external temperatures • some poikilotherms can regulate internal temperatures behaviorally, and are thus considered ectotherms, while homeotherms are endotherms Homeostasis is costly. • As the difference between internal and external conditions increases, the cost of maintaining constant internal conditions increases dramatically: – in homeotherms, the metabolic rate required to maintain temperature is directly proportional to the difference between ambient and internal temperatures Limits to Homeothermy • Homeotherms are limited in the extent to which they can maintain conditions different from those in their surroundings: – beyond some level of difference between ambient and internal, organism’s capacity to return internal conditions to norm is exceeded – available energy may also be limiting, because regulation requires substantial energy output Partial Homeostasis • Some animals (and plants!) may only be homeothermic at certain times or in certain tissues… • pythons maintain high temperatures when incubating eggs • large fish may warm muscles or brain • hummingbirds may reduce body temperature at night (torpor) What are energy units? • 1. umoles of photons per meter squared per second: – umol m-2 s-1 • Watts per meter squared: W m-2 • Sunny day in Colorado: solar input: – 2200 umol m-2 s-1 – 1100 W m-2 – Why no time unit for W? (W = 1 J s-1) • Can you convert between the two units? – Not quite since the conversion depends on wavelength Infrared Light and the Greenhouse Effect 1 • All objects, including the earth’s surface, emit longwave (infrared) radiation (IR). • Atmosphere is transparent to visible light, which warms the earth’s surface. Infrared Light and the Greenhouse Effect 2 • Infrared light (IR) emitted by earth is absorbed in part by atmosphere, which is only partially transparent to IR. • Substances like carbon dioxide and methane increase the absorptive capacity of the atmosphere to IR, resulting in atmospheric warming. Greenhouse Effect - Summary • Greenhouse effect is essential to life on earth (we would freeze without it), but enhanced greenhouse effect (caused in part by forest clearing and burning fossil fuels) may lead to unwanted warming and serious consequences! Ozone and Ultraviolet Radiation • UV “light” has a high energy level and can damage exposed cells and tissues. • Ozone in upper atmosphere absorbs strongly in ultraviolet portion of electromagnetic spectrum. • Chlorofluorocarbons (formerly used as propellants and refrigerants) react with and chemically destroy ozone: – ozone “holes” appeared in the atmosphere – concern over this phenomenon led to strict controls on CFCs and other substances depleting ozone Clouds… • What happens on a cloudy day? – Less radiation comes in… • What happens on a cloudy night? – Less radiation goes out… Plants Respond to Light The Absorption Spectra of Plants • Various substances (pigments) in plants have different absorption spectra: – chlorophyll in plants absorbs red and violet light, reflects green and blue – water absorbs strongly in red and IR, scatters violet and blue, leaving green at depth Plants Respond to Light Photomorphogenesis, Phototropism, Photoperiodism Phytochrome responses (red/far red) flowering and dormancy; branch patterns; root growth Blue light responses stomatal opening; phototropism; chloroplast orientation Photomorphogenesis. – nondirectional, light-triggered development • red light changes the shape of phytochrome and can trigger photomorphogenesis Phototropisms • Phototropic responses involve bending of growing stems toward light sources. – Individual leaves may also display phototrophic responses. • auxin most likely involved Carbon vs. Energy Plants convert LIGHT energy into CHEMICAL energy They use the chemical energy to take CO2 from the atmosphere, and turn it into glucose, and other C-structures…. Seed location? Red light from sun penetrates to seed. Seed germinates. No light from sun to this deep seed. No germination. Red light to seed = near surface Sun Exposure and UV damage • • Sunshine, essential for life, strikes the earth in rays of varying wavelengths. Long rays (infrared) are unseen but felt as heat. Intermediate length rays are visible as light. Shorter rays (ultraviolet) are also invisible and are further divided into the following groups: Ultraviolet (UVA) rays are beneficial in low doses, but may increase the chance of cancer in high doses. UVBs are primarily responsible for sunburn and cancer UVCs are the shortest and most dangerous UV rays contain enough energy to damage DNA in living skin and eye cells. DNA controls the ability of cells to heal and reproduce. The ozone layer allows life to flourish by passing the longer, beneficial wavelengths and effectively blocking almost all UVC, some UVB and a little UVA. The Pigment That Controls Growth and Flowering In Many Plants What Is Phytochrome ? Phytochrome is a pigment found in some plant cells that has been proven to control plant development. This pigment has two forms or “phases” in can exist in. P-red light sensitive (Pr) and P –far red light sensitive (Pfr) forms. The actual plant response is very specific to each specie, and some plants do not respond at all. The structure of Phytochrome A dimer of a 1200 amino acid protein with several domains and 2 molecules of a chromophore. Chromophore 660 nm 730 nm Pr Pfr Binds to membrane Signal Transduction of Phytochrome Membrane Pfr Ga G protein a subunit Pr Guanylate cyclase Ca2+/CaM Calmodulin CAB, PS II ATPase Rubisco FNR PS I Cyt b/f Chloroplast biogenesis cGMP CHS Cyclic guanidine monophosphate bZIP Myb ? Anthocyanin synthesis How Phytochrome Works Light-Regulated Elements (LREs) e.g. the promotor of chalcone synthase-first enzyme in anthocyanin synthesis Promoter has 4 sequence motifs which participate in light regulation. If unit 1 is placed upstream of any transgene, it becomes light regulated. -252 -230 IV III -159 II -131 +1 I Unit 1 5’-CCTTATTCCACGTGGCCATCCGGTGGTGGCCGTCCCTCCAACCTAACCTCCCTTG-3’ Transcription Factors bZIP Myb Light-Regulated Elements (LREs) There are at least 100 light responsive genes (e.g. photosynthesis) There are many cis-acting, light responsive regulatory elements 7 or 8 types have been identified of which the two for CHS are examples No light regulated gene has just 1. Different elements in different combinations and contexts control the level of transcription Trans-acting elements and post-transcriptional modifications are also involved. Which Wavelengths Are Photoperiodic? The length of the night period plays a major role in determining which wavelength will be effective, as the phytochrome pigment tends to revert to Pr during long periods of darkness. R FR Thus the length of exposure to light in a building, or if outdoors, the seasonal light changes, affect how long the plants perceives each form of phytochrome. Photoperiodic Response: It’s all about Preferences! Long Day Plants flower when there is adequate PR Short Day Plants flower when there is adequate Pfr 660 nm 740 nm Pr Synthesis Red Light (Fast) Far Red Light Vegetative (Non-Flowering) Dark Reversion (Slow) Pfr Destruction Reproductive (Flowering) Mid-Summer Sunlight 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Dark Reversion Vegetative (Non-Flowering) (Slow) Pfr Destructio n Reproductive (Flowering) Long-Day Plants Need Low Pr! Long Night 660 nm 740 nm Red Light Pr (Fast) Far Red Light Synthesis Pfr Destruction Dark Reversion Vegetative (Non-Flowering) (Slow) Reproductive (Flowering) Long-Day Plants Need Low Pr! Sunset or Far Red Light 660 nm 740 nm Red Light Pr (Fast) Far Red Light Synthesis Pfr Destruction Dark Reversion Vegetative (Non-Flowering) (Slow) Reproductive (Flowering) Long-Day Plants Need Low Pr! MidSummer Sunlight 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Pfr Destruction Dark Reversion Reproductive (Flowering) (Slow) Vegetative (Non-Flowering) Short-Day Plant Need Low Pfr! Winter Far Red Light 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Pfr Destruction Dark Reversion Reproductive (Flowering) (Slow) Vegetative (Non-Flowering) Short-Day Plant Need Low Pfr! Long Night 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Reproductive (Flowering) Dark Reversion (Slow) Pfr Destruction Vegetative (Non-Flowering) Short-Day Plants Need Low Pfr! Black Cloth 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Dark Reversion Reproductive (Flowering) (Slow) Pfr Destructio n Vegetative (Non-Flowering) Short-Day Plants Need Low Pfr! Night Break 660 nm 740 nm Pr Red Light (Fast) Far Red Light Synthesis Pfr Destruction Dark Reversion Reproductive (Flowering) (Slow) Vegetative (Non-Flowering) Night lighting disrupts reversion to Pr and maintains vegetative status! Light Interruption of Darkness Affects Short- and Long-Day Plants Differently Continuous long, dark period Continuous short, dark period Interrupted dark period Photoperiod type Short-Day (Long-Night) Long -Day (ShortNight) 24-hour day cycle Critical day length The Phytochrome System Works Within The Apical Meristem Photoperiodicresponses are triggered in the meristem (both apical and axillary), long before the new branches develop. We can control development ! To lengthen the night, plants are covered with a blackout shade cloth. Applied in late afternoon and removed in the morning (5 pm to 8 am) Photoperiodic shade cloth Light penetration through the shade cloth should not be more than 2 fc in order to prevent delay in flowering and/or disfigured flowers. SUPPLEMENTAL LIGHTING Light sources. incandescent lamps emit large amounts of red light and are good for lighting mums (standard mum lighting) mums flower when the day length decreases to 13.5 hrs or less whenever the day length is longer than 14.5 hrs plants remain vegetative split each long night in two short nights with supplemental light to prevent flowering DAILY DURATION OF LIGHT The length of day has an effect on two plant processes time of flowering plant maturity This light-induced response is called photoperiodism, and plants that flower under only certain day-length conditions are called photoperiodic. Plants Respond to Gravity • Gravitropism is the response of a plant to the earth’s gravitational field. – present at germination • auxins play primary role – Four steps • • • • gravity perceived by cell signal formed that perceives gravity signal transduced intra- and intercellularly differential cell elongation The pigment phytochrome • Detects R and FR light • Provides information about environment • Answers 3 questions for plant – Am I in the light? – Do I have plants as neighbors or above me? – Is it time to flower? Why bother? • Seeds store materials to start growth • Must reach light before running out of stored materials • Small seeds – Need to be very near surface – Often need light for germination • Germinating plants straighten & open leaves at surface, too Plant neighbors? Red absorbed by other plants. Far red reflected from other plants. Far red enriched = neighbors Why does this matter? • Neighboring plants are threats – Might grow taller, shade you • Solution – Grow at least as tall as neighbors – Need to know that you have neighbors • Isolated plants typically shorter than crowded plants – Other reasons, too Under other plants? Red absorbed by other plants. Far red reflected from other plants or transmitted. Far red enriched = understory Why important? • Best growth strategy for understory plants is different than for plants in open • Need to know whether – Shaded by other plants OR – Just cloudy OR – Late in day (low light) Right time to flower? • Unreliable indicators of time of year – Temperature – Moisture – Light levels • Reliable: length of day/night – Varies with season – Varies with latitude Detected by phytochrome Phytochrome has 2 forms • Red-absorbing phytochrome Pr • Far red absorbing phytochrome Pfr Pr Pfr • Interconverted • Two forms of the same compound • Total amount same In red light Prfr Pr absorbs red light, changes to Pfr form. Pfr Pfr doesn’t absorb red light, stays the same. In far red light Pr Pr doesn’t absorb far red light, stays the same. Prfr Pfr absorbs far red light, changes to Pr form. In pure light Pfr In pure red light, all the phytochrome ends up in the Pfr form. Pr In pure far red light, all the phytochrome ends up in the Pr form. Sunlight Mostly red A little far red In sunlight Pfrr Pfrr Pfr Pfrr Pfr Pfr Pfrr Pfr Pr Pfr Pfrr Pfrr Prfr Pfr Pfr Pfr Pfrr Prfr Pfrr Pfr In sunlight most P gets converted to Pfr form. Start of night Most P in Pfr form. Pfrr Pfrr Pfr Pfrr Pfr Pfr Pfrr Pfr Pr Pfr Pfrr Pfrr Prfr Pfr Pfr Pfr Pfrr Prfr Pfrr Pfr In the dark Pfr form changes gradually to Pr form. Pfrr Pfr Prfr Pfr Pfr Pfrr Prfr Pfrr Pr Pfr Pfrr Pfrr Prfr Pfr Pfr Prfr Pfrr Prfr Pfrr Prfr After a short night Pfr still left. Pfrr Pfr Prfr Pfr Pfr Pfrr Prfr Pfrr Pr Pfr Pfrr Pfrr Prfr Pfr Pfr Prfr Pfrr Prfr Pfrr Prfr LDP = SNP • Needs short night • Needs Pfr still present at end of night • Pfr promotes flowering for LDPs Later in the night More Pfr changes to Pr. Pfrr Prfr Pfr Pfr Pfr Pfrr Pfr Pfrr Prfr Pfrr Prfr Pfrr Pfr Pfr Pr Prfr Pfrr Prfr Pfrr Prfr After a long night All the Pfr is gone. Prfr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Pr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Prfr Day dawns Pfrr Pfrr Pfr Pfrr Pfr Pfr Pfrr Pfr Pr Pfr Pfrr Pfrr Prfr Pfr Pfr Pfr Pfrr Prfr Pfrr Pfr Most P gets converted to Pfr form again. SDP = LNP • Needs long night • Needs Pfr gone at end of night • Pfr inhibits flowering for SDPs LDP SDP Long day: Pfr left at end of short night. Pfr promotes flowering for LDPs. Pfr inhibits flowering for SDPs. Short day: Pfr gone at end of long night. No Pfr to promote flowering for LDPs. No Pfr to inhibit flowering for SDPs. Waiting for the right time • Plants grow leaves until it is time to flower • LDPs wait until the day is long enough – Really night short enough – Some time before June 21 • SPDs wait until the day is short enough – Really night long enough – Some time after June 21 • Flower opening happens later Day neutral plants • Flower when mature enough • Maybe other environmental signals (temp?) • Day length (dark length) doesn’t matter Through the year Specific flowers at specific times. May June September July August October Phytochrome tells plants • • • • If they are near the surface About their plant neighbors Whether it is time to flower And lots more References • http://www.abdn.ac.uk/sms/ugradteaching/GN3502/GN3502_07 32005_1.ppt • http://www.warnercnr.colostate.edu/class_info/by220indy/physical_environment/Physical%20Environment,%20part %202%202004.ppt • http://www.coe.unt.edu/ubms/documents/classnotes/Spring2006/ 256,1,Sensory Systems in Plants • http://128.192.110.246/pthomas/Hort3140.web/Phytochrome%2 0lecture.ppt • http://fp.uni.edu/berg/pp/downloads/PhytochromeAction.ppt • http://www.fsl.orst.edu/~bond/fs561/lectures/radiation.ppt • http://www.coe.unt.edu/ubms/documents/classnotes/Spring2006/ Plant%20Sensory%20Systems%201720_Chapter_40_2005.ppt • http://turfgrass.cas.psu.edu/education/turgeon/CaseStudy/BlueCo urseGreen_01/Blue_Course_Green.ppt • http://siri.uvm.edu/ppt/warmweatherrinjuries/warmweatherrinjur ies.ppt • http://www.cobb.k12.ga.us/~dickerson/ch%2016.ppt