Ch39 PowerPoint LN
advertisement

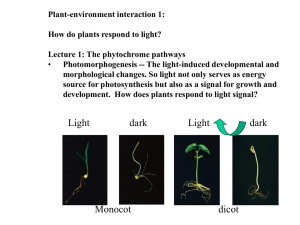
Figure 39.0 A grass seedling growing toward a candle’s light Plant Responses to Internal and External Signals 1 Figure 39.1 Light-induced greening of dark-sprouted potatoes: a dark-grown potato (left), after a week's exposure to natural sunlight (right) 2 Figure 39.2 Review of a general model for signal-transduction pathways 3 Figure 39.3 An example of signal transduction in plants: the role of phytochrome in the greening response (Layer 1) Receptor During transduction we are activating second messengers. In this case it is a G protein that activates cGMP One Pathway Activated 4 Figure 39.3 An example of signal transduction in plants: the role of phytochrome in the greening response (Layer 2) 2nd Pathway Activated 5 Figure 39.3 An example of signal transduction in plants: the role of phytochrome in the greening response (Layer 3) 6 Figure 39.4 Early experiments of phototropism 7 Figure 39.5 The Went experiments Obtain chemical from the tip and store it in an agar block Block, when placed on one side, will cause curvature even when placed in the dark. Block substitutes for the tip. 8 Table 39.1 An Overview of Plant Hormones 9 Polar Auxin Transport 1. Auxin is normally (-) charged. 2. Picks up a H+ and then becomes neutral and can pass through the cell membrane. 3. Within the cell the auxin now ionizes to become A-. 4. Auxin can exit the cell at one specific end where there are carrier proteins 10 Figure 39.6 Polar auxin transport: a chemiosmotic model (Layer 3) 11 Figure 39.7 Cell elongation in response to auxin: the acid growth hypothesis Auxin can cause H+ to be pumped into the cell wall, activating expansins, enzymes that break H bonds of cellulose microfibrils 12 Cytokinins 1. Modified form of adenine (nucleic acid) 2. Plants have cytokinin receptor. One may be at cell membrane and the other within the cytoplasm 3. Act by opening Ca2+ channels. 13 Effects of Cytokinins 1. Produced in roots and will move up the root in xylem. 2. Acting with auxin they will influence: a) cell division b) differentiation 3. Appears that the ratio of auxin / cytokinin is important in just what the exposed cells will do. 4. Apical Dominance: Auxin travels down and suppresses lateral bud growth and the shoot lengthens but no branching. Cytokinins signal the axillary buds to develop. 14 Figure 39.8 Apical dominance: with apical bud (left), apical bud removed (right) Axillary buds are inhibited Cytokinins stimulate axillary bud growth 15 Figure 39.10 Treating pea dwarfism with a growth hormone Effect of Gibberellins: increase in stem elongation in dwarf plants; little response in normal plants Little effect on root growth 16 Figure 39.11 The effect of gibberellin treatment on seedless grapes Thompson seedless grapes: makes grapes grow larger. 17 Abscisic Acid 1. Role in seed dormancy a) High levels of ABA inhibit germination as the seed matures. b) High levels also cause production of proteins that help seed withstand the dehydration conditions of the seed. c) When ABA levels decrease, germination occurs. Levels can decrease by rain, light inactivation or cold inactivation. 2. Drought Stress a) ABA ensures drought survival b) ABA will cause stomata to close rapidly as wilting 18 begins. Huge exodus of potassium from the guard cells. Ethylene Production 1. Plants produce ethylene in response to various stressors: a) drought b) flooding c) mechanical pressure (next slide) d) injury and infection 2. Ripening of fruit 19 Figure 39.13 Ethylene induces the triple response in pea seedlings Ethylene exposure will cause stems to elongate less rapidly, thicken and grow horizontally and this occurs when a seedling encounters objects as it tries to germinate and sprout. 20 Leaf Abscission 1. Leaf loss occurs in the fall to prevent desiccation. The roots cannot absorb water from frozen ground. 2. Abscission layer: base of petiole 3. Enzymes degrade polysaccharides in cell walls 4. All of this is controlled by auxin and ethylene (not abscisic acid) 5. Apoptosis: programmed cell death 21 Figure 39.17 Action spectrum for blue-light-stimulated phototropism Responsible for: 1) phototropisms 2) stomatal opening 3) hypocotyl After 90 minutes 22 Figure 39.18 Phytochrome regulation of lettuce seed germination Phytochromes: another photoreceptor Involved in seed germination There are two forms: Pr and Pfr Red light stimulates germination; Far red light inhibits it. Last flash of light controls the result. 23 Figure 39.19 Structure of a phytochrome One of two domains of the protein Second of two domains of the protein This is the linking of light24 to a chemical response. Figure 39.20 Phytochrome: a molecular switching mechanism 25 Phytochromes and Shade Avoidance • Phytochromes also provide the plant with info about the “quality” of the received light. . . That is, the light’s wavelength. • Eventually the Pr and Pfr reach a dynamic equilibrium. • For a tree that requires lots of light and it is shaded, its level of Pr is high because the canopy is absorbing the red wavelengths of light for PS. • The ratio of Pr to Pfr changes and this induces the plant to use more of its energy to grow taller. • Direct sunlight increases Pfr levels which stimulates branching while inhibiting vertical growth. 26 Figure 39.21 Sleep movements of a bean plant 27 Figure 39.x1 Biological clocks 28 Figure 39.22 Photoperiodic control of flowering 29 Figure 39.23 Reversible effects of red and far-red light on photoperiodic response 30 Figure 39.24 Experimental evidence for a flowering hormone(s) 31 Phytochrome is a molecular switch Switch for: Seed germination, stomatal opening and flowering Phytochrome indicates if light is present It is synthesized in the Pr form And then with light Pr Pfr and the appearance of Pfr is used to detect or indicate the presence of light. Pfr triggers seed germination by activating the genes for alpha amylase production to digest the endosperm of seeds. 32 Overview: Pr equilibrium At night Pfr Pr so Pr increases in concentration and Pfr is degraded. In the daytime Pr Pfr and this marks the end of the dark period. 33 Phytochrome’s Role in Measuring Darkness Phytochrome is the pigment thought to measure the length of night. We know that red light at a wavelength of 660 nm interrupts darkness. That is, red light shortens the night. Therefore, phytochrome must be sensitive to this wavelength. A long night plant fails to flower with exposed to 660 nm (it “breaks up” the long night) A short night plant will flower if a “long night” is interrupted by 660 nm. 34 Phytochrome’s Role in Measuring Darkness A flask of 730 nm or far red cancels the effect of 660 nm. So we “see” this pigment existing in two forms: Pr Pfr Sensitive sensitive to 660 nm to 730 nm 35 Phytochrome’s Role in Measuring Darkness Examples “Mums” or SD/LN Plants: LN is interrupted by 660 nm no flowering (Pr Pfr) LN gets 660, then 730 nm flowering because Pfr Pr So the plant detected “no dark interruption.” 36 Phytochrome’s Role in Measuring Darkness SD / LN Plant Expose to 730 nm and this causes Pfr Pr This maintains a long night situation /environment so flowering occurs. Now expose to 660 nm and Pr Pfr so this is the same as “shortening the night” so no flowering will occur. So it is the last exposure that controls the plants actions. 37 Figure 39.25 The statolith hypothesis for root gravitropism Statoliths are plastids containing starch granules 38 Plant Responses to Environmental Stimuli 1. Responses to Gravity (Gravitropism) a) Place a seedling on its side and: (i) shoot grows upward (- gravitropism) (ii) root grows downward (+ gravitropism) b) Statoliths (i) plastids containing starch that settle to lower portions of cells (ii) this triggers redistribution of auxin (iii) auxin accumulates on lower of shoot and stimulate cell elongation while at the root portion it inhibits growth and causes the upper portion to grow 39 downward. Figure 39.26 Altering gene expression by touch in Arabidopsis Thigmomorphogenesis: touching of stem in a young plant will cause the stem to DECREASE in length. 40 Plant Responses to Envir. Stimuli (cont’d) 2. Thigmomorphogenesis: changes due to touch or pressure (mechanical stress) a) Wind sensed by one side of a tree will cause the trunk to grow thicker b) Rubbing stems of young plants produces shorter plants than controls 3. Thigmotropism: directional growth in response to touch a) vines (ivy) have tendrils that will grow towards something once they touch it. This produces a coiling response. 41 Figure 39.27 Rapid turgor movements by the sensitive plant (Mimosa pudica) 42 Plant Responses to Envir. Stimuli (cont’d) 4. Mimosa plant and wind / touch response. a) collapses and leaflets fold together to prevent water loss, possibly be less conspicuous to herbivores. b) due to loss of turgor in specialized “motor” organs at the joints of the leaf. (i) cells will lose K+ ions, H2O flows out. c) an electrical signal called an action potential can be detected that passes the signal through the leaf. 5. Venus fly-trap and the closing of its leaflets a) Action potentials are transmitted from sensory hairs in the trap to closing mechanism. 43 Plant Responses to Envir. Stimuli (cont’d) 6. Drought Stress: initial sense is too much water lost by transpiration and not enough can be taken up by root hairs. a) Guard cells close b) Increase synthesis of abscisic acid which maintains closure of stomata c) Young leaf growth is inhibited (this decreases surface area for transpiration) d) Roots will grow deeper rather than stay shallow 44 Plant Responses to Envir. Stimuli (cont’d) 7. Flooding a) Water Logged soils (i) lack of oxygen causes ethylene production which induces apoptosis b) Adaptations: some plants (mangroves) have aerial roots that are continuous with submerged roots Some plants, corn, will develop air tubes. 45 Figure 39.28 A developmental response of corn roots to flooding and oxygen deprivation 46 Plant Responses to Envir. Stimuli (cont’d) 8. Salt Stress a) some plants will produce solutes that will counteract the external water potential decrease b) this keeps the internal part of the plant more negative than outside and allows water to continue to flow into the plant. c) halophytes have salt secreting glands or pumps 47 Plant Responses to Envir. Stimuli (cont’d) 9. Heat Stress a) Transpiration and evaporative cooling cannot do it all b) Heat Shock Proteins 10. Cold Stress a) Cell membrane fluidity decreases (i) solute transport through membrane is affected b) Lipid composition is changed as it gets colder (i) increase in unsaturated fatty acids which prevents the packing of fats as temperature drops. c) Solutes and the lowering of freezing point (fpd) d) Some plants accumulate specific solutes to prevent freezing 48 Plant Defense 1. To herbivores: a) thorns (physical defense) b) chemicals (canavanine) (i) Canavanine has a structure similar to the amino acid arginine and therefore canavanine, produced by the plant and ingested by an insect, is incorporated into the insect’s proteins. This alters the shape of the protein. You’re dead. c) Recruitment of organisms against herbivores (i) leaf eaten by certain caterpillars will release volatile chemicals that attract a specific kind of wasp (parasitoid wasp) 49 Plant defense cont’d (ii) Wasps then lay their eggs in the caterpillars (iii) Eggs hatch within caterpillars and eat their way out of the caterpillars and the plant benefits. 50 Figure 39.29 A corn leaf recruits a parasitoid wasp as a defensive response to an herbivore, an army-worm caterpillar 51 Plant defense cont’d 2. To Bacteria a) Phytoalexins: antimicrobial agents that are released upon cell wall damage by the pathogen b) PR (pathogenesis related) proteins: (i) attack the cell wall in the invading bacteria (ii) lets neighboring cells of an invading pathogen and other cells lignify their cell wall to barricade the pathogen. c) Salicylic acid: this is a system-wide, nonspecific activating or warning agent for several days 52 Figure 39.30 Gene-for-gene resistance of plants to pathogens 53 Figure 39.31 Defense responses against an avirulent pathogen 54