PFR design. Accounting for pressure drop
advertisement

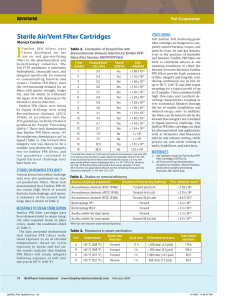
PFR design. Accounting for pressure drop Chemical Reaction Engineering I Aug 2011 - Dec 2011 Dept. Chem. Engg., IIT-Madras Overview • Notation • PFR design equation (mass balance) • Pressure drop equation • Accounts for change in number of moles (due to reaction) • Does not consider phase change • Methodology, with examples • Liquid phase reaction: Calculations are simple • Gas phase reaction: Calculations are more involved Notation • Usual notation applies. – FA = molar flow rate of A, FT = total molar flow rate – V = volume of reactor, Q = volumetric flow rate – D = diameter and A = cross-sectional area of PFR, L = length of PFR, z = distance (between 0 and L) – T = temperature, R = universal gas constant, P = pressure, PA = partial pressure of A – x = conversion, e = fractional increase in number of moles for 100% conversion, for a given feed conditions. – k = rate constant, CA = concentration of A – r = density, m = viscosity – ff = friction factor, Re = Reynolds number – <Vav> = average velocity of the fluid in the PFR. Used sparingly. – Subscript “in” indicates inlet. E.g. FA-in is molar flow rate of A at the inlet. Definitions and formulas The following definitions are of use here: Definition of ‘x’ Definition of ‘e’ Definition of CA FA FAin (1 x) FT FT in (1 e x) FA CA Q For gas phase only: From ideal gas law Q At constant temperature FT RT FT in (1 e x) RT P P FT in (1 e x) RT (1 e x) PinQin Q P P Formulas For liquid as well as gas phase Re D Vav r m 4Qr Dm 32 f f r Q dP dz 2 D5 ff 2 16 if Re < 2100 Re 1 6.9 12.96 log10 Re 2 if Re > 10,000 You don’t need to memorize these formulas PFR design equation • Steady state conditions • For a first order reaction FAin dFA dx FAin rA dV dV FAin dx kC A dV FAin (1 x) FA dx k k dV Q Q • At constant temperature dx (1 x) k (1 x) P k dV Q PinQin (1 e x) dx A k (1 x) P dz PinQin (1 e x) PFR design equation • For any other order of reaction also, write the equation such that dx/dz = f(x,P). i.e. The only unknowns on the RHS must be x and P – i.e. RHS must not have Q or CA. These are not known yet. – But Qin and CA-in are known and hence RHS may have these terms. dx A k (1 x) P (1 x) P , dz PinQin (1 e x) (1 e x) where Ak PinQin • For an ‘n’th order reaction FAnin1 (1 x) n P n dx (1 x) n P n kA n n n n dz Pin Qin 1 e x 1 e x FAnin1 where kA n n Pin Qin Pressure Drop Equation • Under steady state conditions, Reynolds number is a constant – Even though local velocity changes – This is because density also changes with location – We assume that the viscosity of the medium remains the same, even when the reaction occurs • Under steady state conditions, (rQ) = constant Re • Using Re, Calculate the friction factor ff. • Write pressure drop equation 32 f f rQ 2 32 f f rQ Q 32 f f rinQin Q dP 2 D5 2 D5 2 D5 32 f f rinQin Q 32 f f rinQin PinQin (1 e x) dP dz 2 D5 2 D5 P dP (1 e x) dz dz where P 32 f f rinQin PinQin 2 D5 4Qr Dm Solution • Solve both equations simultaneously • Initial conditions: – At z =0, P = Pin – At z = 0, x = 0 • Special case: – When e =0, solve the first equation and find ‘P’. Then substitute for ‘P’ in the second equation and solve for ‘x’ dP (1 e x) dz P 32 f f rinQin PinQin where 2 D5 FAnin1 (1 x) n P n dx (1 x) n P n kA n n n n dz Pin Qin 1 e x 1 e x FAnin1 where kA n n Pin Qin Example • Consider a gas phase reaction under isothermal conditions. (Isomerization) • A B • Pin = 10 atm, Q = 0.005 m3/s, T = 300 K, Pure A is fed, k = 0.1 lit/s, Molecular weight M = 60 g/gmol, Viscosity of the gas = 10-5 Pa-s • What should be the length of the PFR if it is constructed (a) using a 2 cm pipe and the conversion desired is 10%? (b) using a 1.5 cm pipe and the conversion desired is 10% and (c) using a 1.5 cm pipe and the conversion desired is 20% Solution • e= 0. This simplifies the equations dP (1 e x) dz P 32 f f rinQin PinQin where 2 D5 dP 1 dz P P2 Pin2 2 z R=0.08206 atm-lit/(gmol-K) rin Pin M 24.0558 kg/m3 RT Vavin 15.9155 m / s Re 7.65 105 f 0.003 FAin 2.0047 mol/s 1.847 109 Pa2 / m 6.2832 109 1/ ( Pa m) Dia = 2 cm • Conversion and Pressure vs distance Conversion 0.8 0.6 0.4 d = 2 cm e=0 0.2 0 0 10 20 30 40 50 60 70 80 90 100 40 50 60 70 80 90 100 Pressure / Pa 5 10 x 10 9 d = 2 cm e=0 8 7 0 10 20 30 PFR length / m Dia = 2 cm, expanded • Conversion and Pressure vs distance Conversion 0.2 0.15 0.1 d = 2 cm e=0 0.05 0 0 2 4 6 8 10 12 14 16 18 20 8 10 12 14 16 18 20 Pressure / Pa 5 10 x 10 9.9 9.8 d = 2 cm e=0 9.7 9.6 0 2 4 6 PFR length / m Dia = 1.5 cm • 10% conversion is possible, but 20% is not Conversion and Pressure vs distance Conversion 0.2 0.15 0.1 d = 1.5 cm e=0 0.05 0 0 10 20 30 40 50 60 70 30 40 50 60 70 Pressure / Pa 5 10 x 10 5 d = 1.5 cm e=0 0 0 10 20 PFR length / m 2nd order reaction, k = 0.01 • Coupled equations give correct answer Conversion and Pressure vs distance Conversion 0.4 Conversion and Pressure vs distance 0.5 Pressure / Pa 1 2 40 60 80 100 120 140 160 180 x 10 3 4 5 6 7 8 9 10 4 5 6 7 8 9 10 20 40 60 x 10 5 d = 1 cm e=0 0 d = 2 cm e=0 6 0 10 200 0 1 2 3 PFR length / m 80 100 120 140 160 180 200 PFR length / m Conversion and Pressure vs distance 0.4 Conversion 20 8 4 0 5 0 5 10 d = 1 cm e=0 0.1 0.3 0.2 d = 1 cm e = -0.5 0.1 0 0 2 4 6 8 10 12 6 8 10 12 5 10 Pressure / Pa 0 0.2 0 d = 2 cm e=0 Pressure / Pa Conversion 1 0.3 x 10 5 d = 1 cm e = -0.5 0 0 2 4 PFR length / m