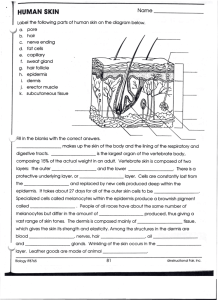
SKIN BIOLOGY
advertisement

SKIN BIOLOGY Alain KHAIAT, Ph.D. Vice President R&D Johnson & Johnson Asia Pacific 1 CONTENTS • Inflammation • Pigmentation • Skin Aging 2 CONTENTS • Inflammation – irritation – sensitization – biochemistry • Pigmentation • Skin Aging 3 EPIDERMIS The cells contained in the epidermis are: • corneocytes • keratinocytes • Langerhans cells 4 DEJ It is the site of adhesion of epidermis to dermis, via: • hemidesmosomes • anchoring filaments (Kalinin) • adhesive protein (Laminin) • fibronectin 5 DEJ Basal cell Hemidesmosome Lamina lucida Anchoring filaments Lamina densa Anchoring fibril 6 DERMIS The dermis contains: • fibrobalsts • mast cells • Langerhans cells • lymphocytes and blood vessels 7 The skin is the interface between the organism and its environment Because it contains: – – – – Langerhans cell lymphocytes blood vessels masts cells exogenous or endogenous stimuli will create inflammation processes 8 INFLAMMATION • Inflammation is the body’s general distress response to biological, physical or chemical causes of: – irritation – sensitization – photosensitization 9 INFLAMMATION • Clinically, inflammation has been defined through 4 signs: – – – – erythema edema pain heat 10 IRRITATION • Irritants are chemical, biological or physical agents which can produce inflammation • Irritation can be either objective or subjective • Objective irritation is characterized by the 4 signs mentioned. It is externally observable • Subjective irritation is characterized by: stinging, burning or itching 11 IRRITATION • The result of insulting the skin is the release of histamine by the mast cells in the irritated area. • Histamine is a potent vasodilator, it produces the visible erythema and increased vascular permeability (leaking of fluid = edema), allowing cells (PMN= polymorphonucleocytes) to migrate to the area 12 SENSITIZATION • Skin sensitization is the result of exposure to sensitizers or allergens • Skin sensitization is a delayed type humoral immune response mediated by the T cell 13 SKIN SENSITIZATION • The sentitizing substance (hapten), combines with a protein in the skin to form the allergen • The Langerhans cells in the stratum germinativum interacts with the allergen and migrates to the lymphoid gland • It then “teaches” the T cells about the allergen 14 SKIN SENSITIZATION • Sensitized T cells migrate to the site and, on contacting the allergen, liberate cytokines • these cytokines attract leukocytes to the site and appear to raise the temperature of the area 15 Langehans cell Allergen cytokine T cell Activated T cell 16 CYTOKINES • Cytokines are essential transmitters of intercellular communication • They have an inherent role in the regulation of responses of the immune system • Each cytokine has multiple functions • More than one cytokine may mediate the same, or very similar, function 17 CYTOKINES • They form part of a complex cellular signaling language • They are proteins 18 T CELL RESPONSE • TYPE 1: cell mediated response, essentially to viruses, bacteria, protozoa, chemicals. Th1 response leads to secretion of: – – – – IL2 IFN TNF IL12 19 T CELL RESPONSE • TYPE 2: humoral response following parasitic infection. Th2 releases: – – – – – IL4 IL5 IL6 IL10 IL13 20 T CELL RESPONSE • The type of response is function of genes and the environment. Th2 Th1 Genes Environment 21 T CELL RESPONSE • Allergic contact dermatitis is in its early stages Th1 (IL2, IFN) becoming later Th2 (IL4). This explains why the reaction decreases • Atopic dermatitis is a Th2: IL4, IL5, IL6, IL10, then IgE, mast cells growth, eosinophil infiltration 22 UV B EFFECT • UV B has been shown to suppress immune reaction (induction phase only) • UV B stimulates synthesis and release of TNF- by keratinocytes which in turn modifies the behavior and morphology of Langerhans cells 23 TWO MECHANISMS • Mast cells can respond directly to external trauma, to antigen-IgE complexes on their surface or to mediators generated from complement (anaphylatoxins) by degranulating and releasing vaso active mediators: histamins • Langerhans cells interact specifically with Tlymphocytes and keratinocytes to initiate host response to antigens 24 BIOCHEMISTRY OF INFLAMMATION • Phospholipids are the major raw material and starting point for the arachidonic acid pathway. Irritants increase the biosynthesis of phospholipids • Arachidonic acid is resident to the cell membrane where it is the source of several major biochemical pathways 25 Phospholipides NSAID Eugenol Phospholipase A2 Steroids Arachidonic Acid Thromboxane Cyclooxygenase Acetylsalycilic acid 12-Lipoxygenase Prostaglandin G2 5-Lipoxygenase Prostacyclin Hydroperoxitetraenoic Acid (HETE) Prostaglandin H2 Leucotrienes Prostaglandins (PGE2, PGF2, etc) 26 ARACHIDONIC PATHWAY • If arachidonic acid is acted upon by cyclooxygenase, prostaglandin G2 is generated. It is itself converted into thromboxane or prostacyclin or PGH2, the later then generating the other members of the PG family. • Thromboxane stimulates platelet aggregation and is a vasoconstrictor 27 ARACHIDONIC ACID PATHWAY • Prostacyclin inhibits platelet aggregation and vasoconstriction • Prostaglandins are non protein chemical mediators: they are fatty acids • 12-lipoxygenase transforms AA into HETE • 5-lipoxygenase catalyses the production of the leukotriens (eicosanoid family) 28 ANTI INFLAMMATORY TESTS • Cytokines secretion by PBL (human peripheral blood lymphocytes) in culture following addition of a stimulant • IL 6 release by human fibroblasts • Contact hypersensitivity in mouse (ear edema), after application of Phorbol ester • Ear edema in mouse following AA inflammation 29 CONTENTS • Inflammation • Pigmentation – anomalies – melanogenesis • Skin Aging 30 PIGMENTATION Skin color is the result of: • nature of the melanin • where the melanin is concentrated, i.e. quantity, type and distribution of melanosomes (epidermis or dermis) • skin vascularisation 31 PIGMENTATION ANOMALIES 1. Melanocytes proliferation is normal: • Freckles: eumelanin zones on pheomelanin backgrounds (skin areas exposed to the sun) • Chloasma: pregnancy mask: hypersecretion of melanin induced by hormonal factors and amplified by the sun 32 PIGMENTATION ANOMALIES • Diffuse brown melanosis: endocrine system disorders or nutritional anomalies • Hypermelanosis can follow cutaneous inflammations: – pigmentation of scars, – caused by irritants combined with sun (photosensitizers like bergamot oil) 33 PIGMENTATION ANOMALIES 2. Melanocytes do not proliferate correctly • Lentigines: can be hereditary, appear anywhere on the body • Solar Lentigo: wider lesion than freckle, occurs after serious sunburn • Senile Lentigo: generally on the back of the hand of older subjects, stimulated by solar exposure 34 PIGMENTATION ANOMALIES • Dubreuilh melanosis or malignant lentigo of the elderly: large pigmented multi colored stain, pre-cancerous • Moles or Naevus: accumulation of melanocytes in epidermis and dermis • Malignant melanomas: cancerous tumors. The first signs are degeneration of existing naevus or Dubreuilh melanosis 35 PIGMENTATION • All methods to reduce pigmentation on the market today have the objective to reduce melanogenesis 36 MELANOGENESIS PATHWAYS L-TYROSINE TYROSINASE 3,4-DIHYDROXYPHENYLALANINE TYROSINASE DOPA QUINONE GSH CYCLISATION GSH-DOPA LEUCODOPACHROME 3-S-CYSTEINYL DOPA DOPACHROME INTERMEDIATE PDTS TRP2 PHEOMELANIN 5,6 DIHYDROXYINDOLE TRP1 QUINONE-IMINE EUMELANIN 37 MELANOGENESIS INHIBITION • Inhibition of the production of active tyrosinase in the ribosomes: placental extract • Inhibition of the transfer of tyrosinase to pre-melanosomes by interrupting glycosylation (tunicamycine, glucosamine) • Elimination of inflammatory reactions (flavonoids, tannins, etc) 38 MELANOGENESIS INHIBITION • Inhibition of tyrosinase: Kojic acid, ascorbic acid, etc. EDTA or Phytic acid (since tyrosinase requires Cu++) • Inhibition of the formation of eumelanin: by adding glutathion and glutathion reductase transforming GSSG into GSH, promote the formation of glutathion DOPA leading to pheomelanin 39 PIGMENTATION • Melanin is formed in the Melanocytes, where it is stored in the melanosomes • Melanocytes extend arms to transfer melanosomes into the keratinocytes • It is the keratinocytes charged with the melanosomes that constitute the dark spots on the skin 40 Pigmentation Formation Mechanism 1 2 Irritation UV • Variety of Causes • Variety of Responses Inflammatory Response KERATINOCYTE (Epidermis) 3 Hormone Tyrosine Melanin MELANOCYTE (Basal Layer) Melanosome Tyrosinase FIBROBLAST Dermis 41 Basic Structure of Skin Stratum Corneum Keratinocyte Viable Epidermis Melanocyte Basal Layer Dermis 42 PIGMENTATION • A novel approach has recently been published: blocking the transfer of melanosomes from the melanocyte to the keratinocytes • Accumulation of charged melanosomes inhibits melanin synthesis 43 SUGGESTED MECHANISM Depigmentation Less melanosome transfer Less eumelanin produced, lighter color 1. Less TRP-1 is made tyrosinase not stable 2. More TRP-2 is made shift to brownish melanins Melanosomes accumulate Negative feed-back 44 NOVEL MECHANISM • Protease Activated Receptor (PAR-2) is expressed in keratinocytes. PAR-2 is activated by trypsin • By inhibiting PAR-2, one probably blocks the keratinocyte-melanocyte interaction • TRP1 (tyrosinase-related protein) decreases leading to less Eumelanin 45 PIGMENTATION TESTING • Tyrosinase activity in solution: mushroom, mouse or human tyrosinase are used with different results • S91 melanoma cells in culture • Keratinocytes-Melanocytes co culture • Guinea pig ear: 15 days treatment • Microswine spotted model: 6-8 weeks 46 PIGMENTATION TESTING • Human volunteers tests: – Chromameter® : L measure – Mexameter® : evaluation of melanin and redness – Photography : visible, UV with data analysis 3 months minimum, changes, so far, are not very significant against placebo 47 CONTENTS • Inflammation • Pigmentation • Skin Aging – skin changes – biochemical changes 48 MANIFESTATIONS OF SKIN AGING • Epidermis : – reduction in cell renewal rate – thickening of stratum corneum – decrease in barrier efficiency : increase in TEWL and hyperkeratosis – ridges are flattened out and intercellular spaces enlarged – pigmentation problems : actinic lentigines – decrease in skin immune system 49 MANIFESTATIONS OF SKIN AGING • Sebaceous glands : – reduction in sebum secretion (hormones influenced) • Sweat glands : – less active • HLP film : – thinning of film means less protective barrier 50 MANIFESTATION OF SKIN AGING • Dermis : – destruction of collagen and elastin fibers network – proteoglycans and glycoproteins are reduced – increase in elastin synthesis : elastosis 51 PHOTOAGING 3 types of reactions to UV exposure: • Free Radicals, essentially due to UVA • Direct cell death, essentially due to UVB • MMP Enzymes 52 FREE RADICALS Free radicals or ROS (reactive oxygen species) can lead to breakage of important molecules: • DNA (mutations, renewal failure, cell death) • collagen, elastin, GAG (skin firmness) • lipids (membrane or structural) 53 UV DAMAGE AND OXIDATIVE STRESS DNA effects Matrix effects DNA fragmentation MMP : TIMP ratio UV damage Membrane effects: ROS Lipids peroxidation Hydroperoxides Enzymatic systems SOD Glutathion peroxidase Heme oxidase 54 DNA DAMAGE • UVA acts through oxidative stress forming “reactive oxygen species” (ROS) that will damage the DNA and lead to cancer 55 DNA DAMAGE • UVB impact on DNA in the cell creating damages which may lead to cancer: nonmelanoma skin cancer (NMSC) 56 57 UVB DAMAGE • Following structural changes in DNA, there is an altered expression of oncogenes and tumor suppression genes, such as p53 • NMSC show a high incidence of mutation in p53 gene 58 59 p 53 GENE Plays an important role in: – blocking the cell cycle after exposure to DNAdamaging agents e.g. UV, in order to allow for repair before duplication – or killing the cell to avoid multiplication of damaged cells (formation of sunburn cells) 60 p 53 GENE The induction of detectable levels of p53 in human epidermis after UV exposure is relevant to skin carcinogenesis 61 Collagen & Photodamage • Major structural component of ECM – 70% of the dry weight of skin • Collagen degradation is believed to play a role in formation of wrinkles 62 Collagen Degradation A balance between MMP:TIMP MMP TIMP 63 MMP ENZYMES TIMP ROS MMP COLLAGEN DEGRADATION 64 MMP ENZYMES • Collagenases (1 to 4) are specific to various collagen, • Gelatinases (A & B) are non specific • Stromelysins (1-3) specific of fibronectin, laminin, collagen IV, etc. • Elastase: elastin • etc 65 MEMBRANE EFFECTS With age, reduction in membrane fluidity leading to less efficient exchanges: – intrinsic: reduction in the methylation of PE into PC – extrinsic: lipid peroxides Methyl donors will restore membrane fluidity 66 ACTIVE PHOTOPROTECTION Replenish antiox system Inhibition of oxidative stress ACTIVE PHOTOPROTECTION Reduce matrix degradation Quench ROS 67 Irradiation of Epidermal Equivalents with Solar Spectrum UV 10-4 Solar Simulator 10-5 W/cm 2 10-6 10-7 10-8 10-9 10-10 10-11 260 280 300 320 340 360 380 Wavelength (nm) MM & TIMP-1 68 400 420 UV Irradiation of Epidermal Equivalents • Markers of damage – MMP-1 induction – TIMP-1 induction, but to a lesser extent than MMP-1 – MMP:TIMP imbalance • Protection provided by – Sunscreens – Anti-oxidants 69 UV Irradiation of Epidermal Equivalents • Model for assessing Photoprotective potential – Botanical ingredients – Fully formulated product 70 THANK YOU 71