Chapter 6 - Victoria College

Chapter 6
The Skeletal System:
Bone Tissue
1
INTRODUCTION
Bone composed of numerous tissue types:
– cartilage
– dense connective tissue
– epithelium
– blood-forming, adipose, & nervous tissues
Each individual bone is an organ
Bone, along with cartilage, makes up skeletal system
Dynamic tissue: ever-changing throughout life
2
Functions of Bone
Support
Protection of soft tissues
Movement
Mineral homeostasis
Blood cell production in red bone marrow
Energy (TG) storage in yellow bone marrow
3
Anatomy of Long Bone
Diaphysis = shaft
Epiphysis = distal & proximal ends
Metaphyses = where epiphysis & diaphysis meet
– epiphyseal plate in growing bone
layer of hyaline cartilage
Articular cartilage over joint surfaces ↓ friction & shock
Medullary cavity = marrow cavity w/in diaphysis
Periosteum = tough membrane covering bone
– enables widthwise growth
– assists in fracture repair
– nourishes bone
– attachmt point for tendons/ligaments
Endosteum = lining of marrow cavity
4
HISTOLOGY OF BONE
Inorganic salts & minerals within matrix
– Hydroxyapatite & calcium carbonate
– Mg +2 , K + , SO
4
-2 , F -
Salts deposited in a framework of collagen fibers during calcification ( mineralization)
– Occurs only in the presence of collagen fibers
– Mineral salts give hardness to bone
– Collagen fibers give tensile strength
5
TYPES OF BONE CELLS
1.
Osteogenic cells
• undergo cell division
• develop into osteoblasts
2.
Osteoblasts = bone-building cells
3.
Osteocytes
• mature bone cells
• principal cells of bone tissue
4.
Osteoclasts
• derived from monocytes
• break down bone tissue (resorption)
6
Bone Matrix
Inorganic mineral salts provide hardness
– hydroxyapatite (CaPO
4
+ Ca(OH)
2
) & CaCO
3
Collagen fibers provide flexibility
– their tensile strength resists being stretched or torn
Bone is not completely solid has small spaces for vessels & red bone marrow
– spongy bone has many such spaces
– compact bone has very few such spaces
7
Compact Bone
Arranged in units called osteons or Haversian systems
Osteons contain:
– blood & lymphatic vessels
– nerves
– osteocytes
– calcified matrix
Osteons aligned in same direction along stress lines
– lines can slowly change as the stresses on bone change
8
Compact Bone
Looks like solid, hard layer of bone
Shaft of long bones & external layer of all bones
Resists stresses produced by weight & movement
9
Histology of Compact Bone
Osteon = concentric rings (lamellae) of calcified matrix surrounding vertically oriented blood vessel
Osteocytes found in lacunae
Osteocytes communicate through canaliculi filled with ECF
– Canaliculi connect cells
Interstitial lamellae represent older osteons that were partially removed during bone remodeling
10
Spongy Bone
Does not contain osteons
Consists of trabeculae surrounding many red marrow-filled spaces (Figure 6.3b)
Most of structure of short, flat, & irregular bones
Epiphyses of long bones
Lightweight
Supports & protects red bone marrow
11
Trabeculae of Spongy Bone
Oriented along lines of stress & where stress applied from many directions, but not in areas of high stress
Spaces between trabeculae are filled with red marrow where blood cells develop
Lacunae contain osteocytes
– Directly nourished by blood in medullary cavities
12
Blood & Nerve Supply of Bone
Highly vascularized & innervated
Periosteal arteries enter diaphysis thru Volkmans canal
– supply periosteum
Nutrient arteries
– enter thru nutrient foramen in center of diaphysis
– supply compact bone of diaph., spongy bone,
& red marrow up to epiphys. plates
Metaphys & epiphyseal arteries
– enter thru meta-/epiphysis
– supply red marrow & bone tissue of meta-
/epiphyses 13
Blood Supply c’td
Veins carry blood away from long bones
– Nutrient veins accompany nutr. artery in diaphy.
– Numerous epiphyseal/metaphyseal veins
exit thru epiphyses
– Periosteal arteries exit thru perisoteum
Nerves accompany blood vessels
– Sensory nerves in periosteum
REMEMBER: Arteries carry oxygenated blood FROM the heart to the tissues (bone)
& veins carry deoxygenated blood from the tissues TO the heart.
14
BONE FORMATION
Osteogenesis or ossification
Begins when mesenchymal cells provide template for subsequent ossification
Two types of ossification:
– Intramembranous ossification = formation of bone directly within mesenchyme
arranged in sheetlike layers
resemble membranes
– Endochondral ossification = formation of bone from hyaline cartilage that develops from mesenchyme
15
Intramembranous Ossification
Forms flat bones of skull and mandible
1) Formation of ossification center
• mesenchymal cells differentiate into osteogenic cells
& then into osteoblasts
• osteoblasts secrete XC matrix until surrounded by it
2) Calcification
• matrix secretion stops
osteoblast becomes osteocyte
• Ca & other minerals deposited calcification
16
Intramembranous Ossification (c’td)
3) Trabeculae formation
• XC matrix centers join to form bridges of trabeculae that constitute spongy bone
• blood vessels fill spaces btwn trabeculae
• CT develops into red bone marrow
4) Formation of periosteum
• mesenchyme condenses at outer edge & becomes periosteum
• thin layer of compact bone covers spongy bone center
17
Endochondral Ossification
Replacement of cartilage by bone
Forms most bones of the body
1) Formation of cartilage model
• Mesenchymal cells cluster in shape of future bone & develop into chondroblasts
• Chondroblasts secrete cartilage ECM form cartilage model
• Perichondrium formation
2) Growth of cartilage model
• Chondrocytes continually divide growth in length
(interstitial)
• Addition of ECM from chondroblasts in perichond.
growth in thickness (appositional)
• Chondrocytes in mid-region hyptertrophy & calcify
• Other chondrocytes die form lacunae 18
Endochondral Ossification
3) Development of Primary Ossification Center
– proceeds inward from external surface of bone
– nutrient artery stimulates differentiation of osteogenic cells in perichondrium into osteoblasts
penetrates perichondrium thru ctr of cartilage model
– perichondrium becomes periosteum
– @ ctr of model, periosteal capillaries grow & stimulate growth of prim. ossific. ctr
where bone will replace most of cartilage
– osteoblasts deposit bone matrix over calcified cartilage
form spongy bone trabeculae
19
Endochondral Ossification
4) Development of medullary cavity
• Osteoclasts break down some of newly formed spongy bone trabeculae
• Resultant cavity in diaphysis becomes medullary (marrow) cavity
• Wall of diaphysis eventually replaced by compact bone
20
Endochondral Ossification
5) Development of secondary ossification center
– Formed @ birth when epiphyseal artery enters epiphyses
– Spongy bone remains @ center of epiphyses
No formation of medullary cavity (as in 1 ° ossification)
– Proceeds outward from center of epiphysis to outer surface
6) Formation of articular cartilage & growth plates
– Hyaline cartilage covering epiphyses becomes articular cartilage
– Epiphyseal plates responsible for lengthwise growth of long bones
When growth plates “close,” growth stops
21
Bone Scan
Radioactive tracer is given intravenously
Amount of uptake is related to amount of blood flow to the bone
“Hot spots” are areas of increased metabolic activity that may indicate cancer, abnormal healing or growth
“Cold spots” indicate decreased metabolism of decalcified bone, fracture or bone infection
22
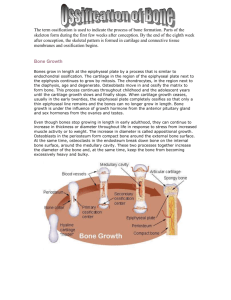
BONE GROWTH
Two types
– Appositional (↑ in thickness)
All bones
– Lengthwise
Long bones
23
Growth in Length
Epiphyseal plate consists of four zones:
1) Zone of resting cartilage
• Nearest epiphysis
• Scattered chondrocytes
Do not function in bone growth
Anchor epiphyseal plate to epiphysis
2) Zone of proliferation cartilage
• Chondrocytes divide replace dying chondrocytes
3) Zone of hypertrophic cartilage large chondrocytes
24
Growth in Length
4) Zone of calcified cartilage
• Dead chondrocytes ECM has calcified
• Osteoclasts dissolve calcified cartilage
• Osteoblasts lay down bone ECM
Replace calcified cartilage become “new diaphysis”
Activity of epiphyseal plate = only means by which diaphysis can increase in length
– New chondrocytes formed on epiphys side of plate
– Old chondrocytes on diaphys side replaced by bone
– Thickness remains constant, growth in length occurs
25
Bone Growth in Length
Closure of epiphyseal plate = completion of growth in length
– Epiphyseal line forms
– Damage to epiphyseal plate accelerates closure
Between ages 18 & 25, epiphyseal plates close
– Females: about age 18
– Males: about age 21
26
Growth in Thickness
Appositional growth
Four steps in this process:
1) Periosteal cells differentiate into osteoblasts
secrete collagen fibers & other substances
osteoblasts become surrounded by ECM osteocytes
2) Ridges fuse to form canal for blood vessels
periosteum becomes endosteum
3) New concentric lamellae formed when osteoblasts in endosteum deposit bone ECM inward
4) As new osteon forms, osteoblasts under periosteum form new outer lamellae
Repetition of step 2 growth continues
27
Bone Remodeling
Ongoing replacement of old bone tissue w/ new bone tissue
– Resorption: osteoclasts destroy old bone
– Deposition: osteoblasts construct new bone
– @ any given time, 5% of total bone mass remodeled
Renewal varies w/ bone type & location within body
Removes injured bone
Triggered by a number of factors
– Bone strength related to degree by which it is stressed
Lifting weights strengthens bone thickens it
Controlled by hormones, vitamins & minerals
28
Bone Remodeling
Resorption
– Osteoclasts attach to endosteum/periosteum & form leak-proof seal around cell edges
– Secrete lysosomal enzymes & acids
enzymes digest collagen
acids dissolve bone minerals
– Degraded proteins, Ca & P released into interstitial fluid diffuse into capillaries
Deposition
– Osteoclasts depart site of remodeling
– Osteoblasts begin new bone deposition
29
Factors Affecting Bone Growth
Nutrition
– Minerals
primarily Ca and P for bone growth
also F, Mg, Fe & Mn in lesser quantities
– Vitamins
vitamin C for collagen formation
vitamins K and B
12 for protein synthesis
vitamin A activates osteoblasts
30
Factors Affecting Bone Growth
Sufficient hormones
– During childhood
insulinlike growth factor (IGF) most important
– promotes cell division at epiphyseal plate
– enhance protein synthesis
– stimulated by human growth hormone (hGH)
– Thyroid hormones (T
3
– Estrogens & androgens
&T
4
)
@ puberty: stimulate sudden growth
– ↑ osteoblast activity & synthesis of bone ECM
“growth spurt”
– modifications of skeleton characteristic of male/female forms
adulthood: contribute to remodeling
– Vitamin D, parathyroid hormone (PTH) & calcitonin
31
Fracture and Repair of Bone
A fracture = any break in bone
4 steps to fracture repair (Figure 6.10)
1) Formation of fracture hematoma
• Blood leaks from vessels crossing fracture line clot forms
• Nearby bone cells die swelling & inflammation occur
• Phagocytes & osteoclasts remove damaged bone tissue
• May last up to several weeks
2) Fibrocartilaginous callus formation
• Mass of repair tissue consisting of collagen fibers & cartilage
Bridges broken ends of bone
Secreted by blast cells in periosteum
• Takes about 3 weeks
32
Fracture and Repair of Bone
3) Bony callus formation
• Osteogenic cells differentiate into osteoblasts
Well-vascularized tissue
Form spongy bone trabeculae
• Trabeculae join living & dead parts of bone
• Fibrocartilage converted into spongy bone bony callus
• 3-4 months
4) Bone remodeling
• Osteoclasts gradually resorb dead portions of fragments
• Compact bone replaces spongy bone @ periphery of fracture
• Usually a thickened area remains on bone surface evidence of break
33
Fractures
Named for shape or position of fracture line
Common types of fracture
– greenstick -- partial fracture
– impacted -- one side of fracture driven into the interior of other side
34
Fractures
Named for shape or position of fracture line
Common types of fracture
– closed -- no break in skin
– open fracture --skin broken
– comminuted -- broken ends of bones are fragmented
35
Fractures
Named for shape or position of fracture line
Common types of fracture
– Pott’s -- distal fibular fracture
– Colles’s -- distal radial fracture
– stress fracture -- microscopic fissures from 36 repeated strenuous activities
Calcium Homeostasis & Bone Tissue
99% of total body calcium found in bone
Ca +2 ions required by several body systems
– nerve & muscle cell function
– blood clotting
– enzyme function in many biochemical reactions
Plasma level maintained @ 9-11mg/100mL
– Bone helps maintain plasma [Ca +2]
balance rates of deposition & resorption
– Small changes in blood levels of Ca +2 can be deadly
cardiac arrest if too high
respiratory arrest if too low 37
Hormonal Influences
Parathyroid hormone (PTH) is secreted if Ca +2 levels falls
– PTH gene turned on more
PTH secreted from gland
– osteoclast activity increased
– kidney retains Ca +2
– stimulates calcitriol production
Calcitonin secreted from parafollicular cells in thyroid if
Ca +2 blood levels get too high
– inhibits osteoclast activity
– increases bone formation by osteoblasts
38
Parathyroid hormone acts to RAISE blood
Ca +2 levels
Calcitonin works to DECREASE blood Ca +2 levels
39
EXERCISE AND BONE TISSUE
Ability to alter its strength in response to mechanical stress
– increased deposition of mineral salts & production of collagen fibers
– removal of mechanical stress leads to weakening of bone through demineralization
(loss of bone minerals) and collagen reduction.
reduced activity while in a cast
astronauts in weightless environment
bedridden person
– Weight-bearing activities help build & retain bone mass 40
AGING AND BONE TISSUE
loss of calcium and other minerals from bone matrix (demineralization)
– may result in osteoporosis
– very rapid in women 40-45 as estrogens levels decrease
– in males, begins after age 60
decreased rate of protein synthesis
– decrease in collagen production which gives bone its tensile strength
– decrease in growth hormone
– bone becomes brittle & susceptible to fracture
41
Osteoporosis
Decreased bone mass resulting in porous bones
Those at risk
– white, thin menopausal, smoking, drinking female with family history
– athletes who are not menstruating due to decreased body fat & decreased estrogen levels
– people allergic to milk or with eating disorders whose intake of calcium is too low
Prevention or decrease in severity
– adequate diet, weight-bearing exercise, & estrogen replacement therapy (for menopausal women)
42
– behavior when young may be most important factor
Disorders of Bone Ossification
Rickets
calcium salts are not deposited properly
bones of growing children are soft
bowed legs, skull, rib cage, and pelvic deformities result
Osteomalacia
new adult bone produced during remodeling fails to ossify
hip fractures are common
43