Answer
advertisement

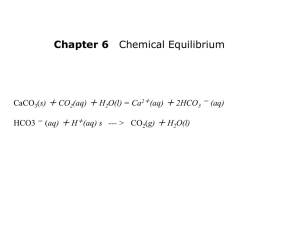
JEOPARDY Stoichiometry, Atomic/Molecular Structure Energetics, Hess’s Law Acids and Bases Gas Law Miscellaneous 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 100: STOICHIOMETRY, ATOMIC/MOLECULAR STRUCTURE How many atoms are in 23 g of potassium? Answer 200: STOICHIOMETRY, ATOMIC/MOLECULAR STRUCTURE A sheet of paper has a density of 0.2 g/cm3, a surface area of 93.5 in2, and a weight of 2.0 mg. What is the thickness of the sheet? Answer 300: STOICHIOMETRY, ATOMIC/MOLECULAR STRUCTURE Fill in the following table: Symbol Protons 27Al 43 Electrons Neutrons Mass Number Answer 37 55 48 400: STOICHIOMETRY, ATOMIC/MOLECULAR STRUCTURE Which reagent is the limiting reactant when 0.450 mol Al(OH)3 and 0.450 mol H2SO4 are allowed to react? Answer 500: STOICHIOMETRY, ATOMIC/MOLECULAR STRUCTURE Write the following names of the compounds: Potassium Phosphide Calcium Hydride Lithium Phosphate Answer 100 STOICH. ANSWER 3.5 x 1023 atoms of potassium 200 STOICH. ANSWER 300 STOICH. ANSWER Symbol 27Al 98Tc 85Rb Protons 13 43 37 Electrons 13 43 37 Neutrons 14 55 48 Mass Number 27 98 85 400 STOICH. ANSWER 500 STOICH. ANSWER Potassium Phosphide= K3P Calcium Hydride= CaH2 Lithium Phosphate= Li3PO4 100: ENERGETICS 2 O3(g) 3O2 (g) ΔH= -284.6 kJ What is the enthalpy change for this reaction per mole of O3? Answer 200: ENERGETICS 2 CH3OH 2 CH4 + O2 ΔH= 252.8 kJ How much heat is transferred when 24.0 g of CH3OH is decomposed by this reaction at constant pressure? Answer 300: ENERGETICS The specific heat of octane is 2.22 J/g-K. How many J of heat are needed to raise the temperature of 80.0 g of octane from 13-60 °C? Answer 400: ENERGETICS Using Hess’s Law: Find the ΔH for the reaction PCl5(g) → PCl3(g) + Cl2(g), given the following reactions: P4(s) + 6Cl2(g) → 4PCl3(g) ΔH = -2439 kJ 4PCl5(g) → P4(s) + 10Cl2(g) ΔH = 3438 kJ Answer 500: ENERGETICS Find the ΔH for the reaction 2CO2(g) + H2O(g) → C2H2(g) + 5/2O2(g), given the following reactions: C2H2(g) + 2H2(g) → C2H6(g) ΔH = -94.5 kJ H2O(g) → H2(g) + 1/2O2 (g) ΔH = 71.2 kJ C2H6(g) + 7/2O2(g) → 2CO2(g) + 3H2O(g) ΔH = -283 kJ Answer 100 ENERGETICS ANSWER ΔH= -142.3 kJ/mol O3 200 ENERGETICS ANSWER 300 ENERGETICS ANSWER q= mcΔT q= (80.0 g)(2.22 J/g-°C)(60-13°C) q= 8350 J 400 ENERGETICS ANSWER 500 ENERGETICS ANSWER 100: ACIDS AND BASES What is the pH of a 0.04 M HCl solution? Answer 200: ACIDS AND BASES In the following equation, lable the acid, base, conjugate acid and conjugate base. HC2H3O2 (aq) + H2O (l) H3O+ (aq) + C2H3O2- (aq) Answer 300: ACIDS AND BASES What is concentration of hydroxide ions if the pH is 8.9? Answer 400: ACIDS AND BASES Find the pH of a solution containing 0.33 M Acetic Acid (HC2H3O2). The Ka value for acetic acid is 1.8 x 10-5. HC2H3O2 ⇔ C2H3O2- + H+ Answer 500: ACIDS AND BASES Caffeine is a base with a formula C8H10N4O2. If there is 0.0032 g of caffeine in a solution of 1 L, what will the pH of the solution be? Caffeine has a Kb of 4.1 x 10-4. Answer 100 ACIDS/BASES ANSWER pH= -log (.04)= 1.40 200 ACIDS/BASES ANSWER HC2H3O2 (aq) + H2O (l) H3O+ (aq) + C2H3O2- (aq) HC2H3O2= acid H2O= base H3O+= conjugate acid C2H3O2-= conjugate base 300 ACIDS/BASES ANSWER What is concentration of hydroxide ions if the pH is 8.9? 14 - 8.9= 5.1= pOH [OH-]= 10-5.1= 7.9 x 10-6 M 400 ACIDS/BASES ANSWER 1.8 x 10-5= x2/0.33 x= 0.0024= [H+] -log(.0024)= 2.61 500 ACIDS/BASES ANSWER 0.0032 g * (1 mol/166.18 g)= 1.92 x 10-5 mol/ 1L= 1.92 x 10-5 M 4.1 x 10-4= x2/ 1.92 x 10-5 x= 8.9 x 10-5 = [OH-] pOH= -log (8.9 x 10-5 )= 4.05 14 - pOH= pH= 9.95 100: GAS LAW What are the conditions of STP? Answer 200: GAS LAW If 0.03 mol of gas is in a 250 mL container at 450 K, what will the pressure be? Answer 300: GAS LAW What volume is occupied by 2.1 g of He at a pressure of 5.6 atm at 298 K? Answer 400: GAS LAW If a gas has a pressure of 4.5 atm in a 1 L container and you transfer it to a 2 L container, what will the new pressure be? Answer 500: GAS LAW If a gas is in a 520 mL container at 298 K and you raise the temperature to 450 K, what volume will the gas occupy? Answer 100 ANSWER GAS LAW Standard Temperature and Pressure: 1 atmosphere, 273 K 200 ANSWER GAS LAW If 0.03 mol of gas is in a 250 mL container at 450 K, what will the pressure be? PV=nRT P(0.250 L)= (.03 mol)(0.0821L*atm/mol*K)(450 K) P=4.4 atm 300 ANSWER GAS LAW 2.1 g He*(1 mol/4 g He)= 0.525 mol He PV=nRT (5.6)V= (.525)(0.0821)(298) V= 2.3 L 400 ANSWER: GAS LAW P1V1= P2V2 (4.5 atm)(1 L)= P2 (2 L) P2= 2.25 atm 500 ANSWER GAS LAW V1/T1=V2/T2 (520 mL)/(298 K) = V2/ (450 K) V2= 785 K 100: MISC. The electron configuration [Ar]4s23d104p2 represents what element? Answer 200: MISC. Draw the Lewis Structure of BeF2 Answer 300: MISC What is the wavelength of a radiation that has a frequency of 7.9 x 10-5 seconds? Answer 400 MISC. Write all the possible quantum (n, l, ml, ms) numbers for n=2. Answer 500 MISC. What is the value of the rate constant, k, for the reaction? 2A + B2 2 AB Answer 100 ANSWER MISC. Ge 200 ANSWER MISC. 300 ANSWER MISC. c= λν 3 x 108 m/s= λ (7.9 x 10-5 s) λ= 3.8 x 1012 m 400 ANSWER MISC. n= 2 l= 0, 1 For l= 0 ml= 0 and ms= ±1/2 For l= 1, ml= -1, 0, or 1 and ms= ±1/2 for each of the ml values 500 ANSWER MISC. • Trial 12: A stays same, B2 increases by x 4 and rate increases by x 4 so B2 is order 1 • Trial 1 3: A halves, B2 stays same, rate stays same so A is 0 order • Rate= k[B2] • 0.34 M/s= k (0.43M) k=0.79 s-1