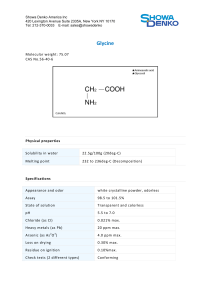
NH 3 + Glycine = CH 2 -COO - General Biochemistry Homepage
advertisement

Chapter 2 : Water Learning goals: • What kind of interactions occur between different type of molecules in water. • Why water is a good medium for life • Why nonpolar moieties aggregate in water • How dissolved molecules alter properties of water • How weak acids and bases behave in water: to be able to solve weak acid problems with the Henderson-Hasslebalch equation. • How buffers work and why we need them • How water participates in biochemical reactions Structure ICE Common H-bonds in Biochemistry Some Biologically Important H-bonds H-bond Strength and Alignment Importance of Hydrogen Bonds • • • • • • • Source of unique properties of water Structure and function of proteins Structure and function of DNA Structure and function of polysaccharides Binding of substrates to enzymes Binding of hormones to receptors Matching of mRNA and tRNA “I believe that as the methods of structural chemistry are further applied to physiological problems, it will be found that the significance of the hydrogen bond for physiology is greater than that of any other single structural feature.” –Linus Pauling, The Nature of the Chemical Bond, 1939 Water as a Solvent • Water is a good solvent for charged and polar substances – amino acids and peptides – small alcohols – carbohydrates • Water is a poor solvent for nonpolar substances – nonpolar gases – aromatic moieties – aliphatic chains Solvation and Hydration Spheres Flickering Clusters and Clathrate Cages The Hydrophobic Effect • Refers to the association or folding of nonpolar molecules in the aqueous solution • Is one of the main factors behind: – protein folding – protein-protein association – formation of lipid micelles – binding of steroid hormones to their receptors • Does not arise because of some attractive direct force between two nonpolar molecules Substrates Must Displace Water to Bind Enzymes Approximate Bond Strength, kJ/mole Distance, nm 12-30 0.3 20 0.25 <40 - 0.4 – 4.0 0.2 Water Bound to Hemoglobin Hb purified from water Hb with Water Removed Proton Hop and Hydronium Water Bound in a Protein Channel (Cytochrome f) Facilitates Proton Hopping – see later in Photosynthesis Osmotic Pressure Cell Response to Osmotic Pressures Plants Use Osmotic Pressure Plants Use Osmotic Pressure Protection Against Wind Ionization of Water Keq = [H+][OH-] / [H2O] = 1.8 x 10-16 M Concentration of water - one liter = 1,000g Mole Wt Water = 18.015 [H2O] = 55.5 M Kw = [H+][OH-] = Keq x [H2O] = 1 x 10-14 M2 for pure water [H+] = [OH-] so, [H+] = 10-7 M pH is negative log [H+] , for pure water = 7.0 Weak Acids HA ↔H + + A- K e = [H+][A-] / [HA] = Ka Henderson-Hasselbalch Equation Rearranges Ka pH = pKa + log ( [A-] / [HA] ) when pKa = pH … [A-] = [HA] A Weak acids have different pKas Enzymes have pH optima Related to their Function Water as a Reactant Problem 18 in Chapter 2 1 liter of 0.1 M glycine. a. what pH’s is glycine a good buffer due to its amino group: Problem 18 in Chapter 2 1 liter of 0.1 M glycine. a. What pH is glycine a good buffer due to its amino group: NH3+ Glycine = CH2-COOpKa’s = 2.34 and 9.6 So it would be good +/- 1.0 from each pKa which would be from 1.3 to 3.3 and 8.6 to 10.6 for the amino group. Problem 18 in Chapter 2 NH3+ Glycine = CH2-COO- I liter of 0.1 M glycine. pKa’s = 2.34 and 9.6 b. in a 0.1 M solution, pH 9.0 what fraction has the amino group as –NH3+ ? Example of a Clicker Question: R-NH3+ is A. HA B. AC. H2O D. H+ E. OH- Problem 18 in Chapter 2 NH3+ Glycine = CH2-COOpKa’s = 2.34 and 9.6 I liter of 0.1 M glycine. b. in a 0.1 M solution, pH 9.0 what fraction has the amino group as –NH3+? pH = pKa + log A/HA 9.0 = 9.6 + log A/HA log A/HA = -0.6 HA + A = 0.1M A/HA = 0.25 0.25HA = A so HA + 0.25HA = 0.1 M :: 1.25HA = 0.1M so HA = 0.08 M…and that is 80% of 0.1M so not asked: A = 0.02 M or 20% Problem 18 in Chapter 2 NH3+ Glycine = CH2-COOpKa’s = 2.34 and 9.6 I liter of 0.1 M glycine. c. How much 5M KOH is needed to change pH from 9 to 10 for 1 Liter of 0.1M glycine? Problem 18 in Chapter 2 NH3+ Glycine = CH2-COOpKa’s = 2.34 and 9.6 I liter of 0.1 M glycine. c. How much 5M KOH is needed to change pH from 9 to 10 for 1 Liter of 0.1M glycine? pH = pKa + log A/HA 10 = 9.6 + log A/HA so: log A/HA = 0.4 thus A/HA = 2.5 2.5HA = A HA + A = 0.1 M HA + 2.5 HA = 0.1 M 3.5HA= 0.1M so HA at pH 10 = 0.029 moles/L from pH 9 HA is converted to A by adding OH-, that is HA is lowered from 0.08M to 0.029M or a change of 0.051 moles 0.051 moles/5 moles/L = 0.01 L 10 ml of 5M KOH Problem 18 in Chapter 2 NH3+ Glycine = CH2-COO- I liter of 0.1 M glycine. pKa’s = 2.34 and 9.6 d. When 99% of glycine is in its –NH3+ form, what is the pH of solution due to it’s amino group? (functionally reworded from the text) pH = pKa + log A/HA so this is easy HA dominates, so it will be on the acid side of the pKa. A is only 1% or 0.01 so the log of A/HA ≈ -2 thus pH = 9.6 – 2 = or 7.6 Examples of Written Exercise Topics – Choose One 1. Make two arguments: one for and one against the existence of H3PO4 at pH 12.5 (phosphate has three pKa’s: 2.14, 6.86, 12.4). 2. Acetate is not charged at its isoelectric point when it has a charge of zero. Explain. 3. Glycine is charged at its isoelectric point when it supposedly has a charge of zero. What is going on? Things to Know and Do Before Class 1. General Chemical Properties of Water. 2. pH definition and what it means+how to calculate it. 3. Strong vs Weak Acids. 4. Henderson-Hasselbalch Equation and how to do calculations with it. 5. Weak bonds and their relative bond strength. 6. Make sure you are able to do EOC Problems calculating pH (2-5, 8), pH affects solubility (14) and uptake of aspirin (15) and rest on buffers (11): They are part of Class Clicker Questions and Case Study (aspirin).