Amino Acid Titration: Isoelectric Point Determination
advertisement
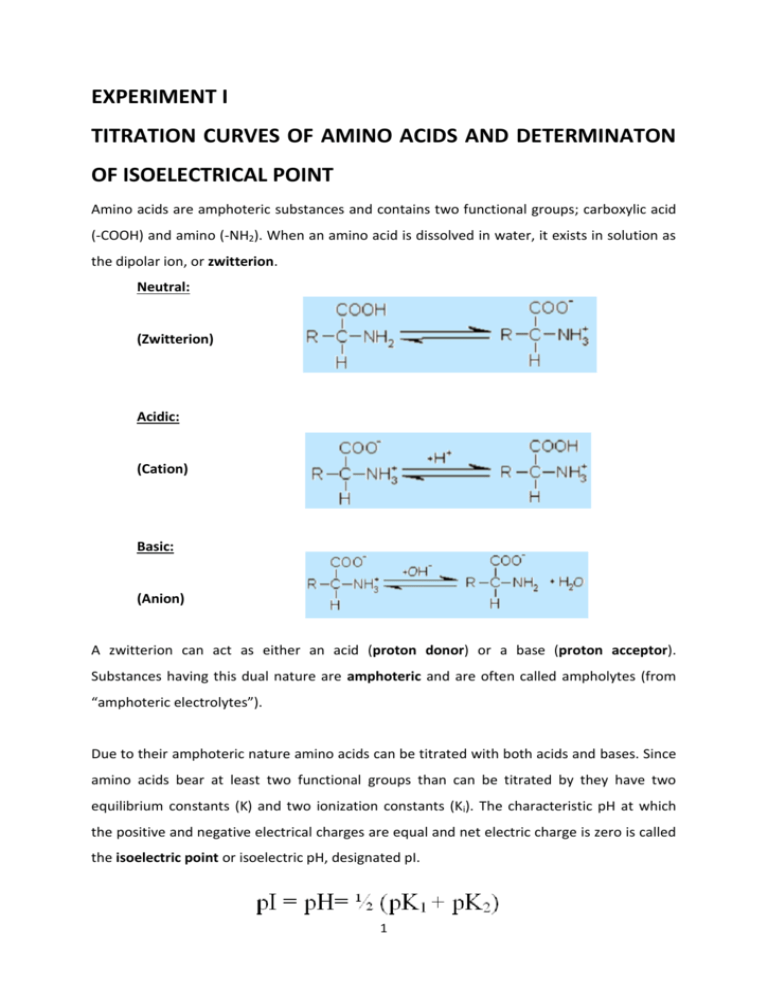
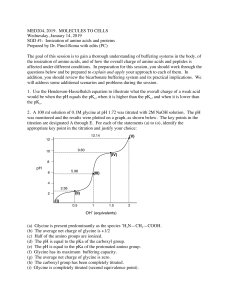
EXPERIMENT I TITRATION CURVES OF AMINO ACIDS AND DETERMINATON OF ISOELECTRICAL POINT Amino acids are amphoteric substances and contains two functional groups; carboxylic acid (-COOH) and amino (-NH2). When an amino acid is dissolved in water, it exists in solution as the dipolar ion, or zwitterion. Neutral: (Zwitterion) Acidic: (Cation) Basic: (Anion) A zwitterion can act as either an acid (proton donor) or a base (proton acceptor). Substances having this dual nature are amphoteric and are often called ampholytes (from “amphoteric electrolytes”). Due to their amphoteric nature amino acids can be titrated with both acids and bases. Since amino acids bear at least two functional groups than can be titrated by they have two equilibrium constants (K) and two ionization constants (Ki). The characteristic pH at which the positive and negative electrical charges are equal and net electric charge is zero is called the isoelectric point or isoelectric pH, designated pI. 1 Acid-base titration involves the gradual addition or removal of protons. Figure shows the titration curve of the diprotic form of glycine. The plot has two distinct stages, corresponding to deprotonation of two different groups on glycine. The shaded boxes, centered at about pK1=2.34 and pK2=9.60, indicate the regions of greatest buffering power. Glisin can be used as a buffer solution at +/- 1 pH range of these pK values. That means glycine has two regions of buffering power. At pH 5.97, the point of inflection between the two stages in its titration curve, glycine is present predominantly as its dipolar form, fully ionized but with no net electric charge, which is the pI of glycine. Amino acids with an ionizable R group have more complex titration curves, with three stages corresponding to the three possible ionization steps 2 CHEMICALS HCl, NaOH, Glycine METHOD Prepare 50 mL of 0.1 N Glycine. After calibrating the pH meter, pour 20 mL of glycine solution to a beaker and determine the pH of the solution. Titrate your solution with 0.1 N HCl. After each addition of 500 µL of acid mix the content of the beaker vigorously and determine pH. Continue this process until there is no discernible change of the pH of the solution. Wash and dry the electrode of the pH meter. Pour 20 mL of glycine solution to another beaker and determine the pH of the solution. Titrate your solution with 0.1 N NaOH as described above. Write down the data you obtain in data sheet. Acid-Base titration data sheet: 3 CALCULATION Draw the graph between added acid or base volume (mL) to pH on a graph paper (milimetric paper). Using the graph determine pKa, pKb and pI values for glycine. Compare your findings with the theoretical values for glycine. QUESTIONS 1- How do you calculate the pI value of glutamic acid and histidine? What is the importance of different pI values of amino acids? 4