slides
advertisement
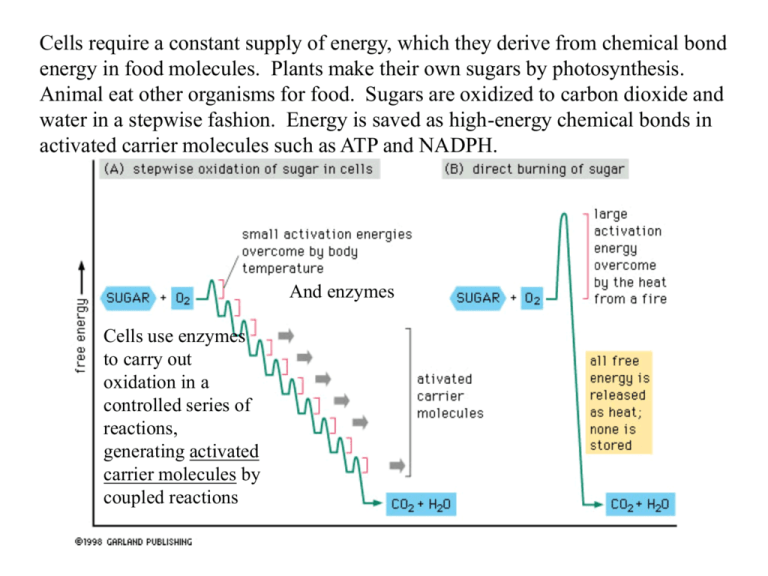
Cells require a constant supply of energy, which they derive from chemical bond energy in food molecules. Plants make their own sugars by photosynthesis. Animal eat other organisms for food. Sugars are oxidized to carbon dioxide and water in a stepwise fashion. Energy is saved as high-energy chemical bonds in activated carrier molecules such as ATP and NADPH. And enzymes Cells use enzymes to carry out oxidation in a controlled series of reactions, generating activated carrier molecules by coupled reactions Enzymatic breakdown (digestion) occurs in our intestine outside cells or in lysosomes inside cells Glycolysis = a chain of reactions which converts glucose (6 carbons) into 2 pyruvate (3 carbons) Large polymeric molecules are broken down into their monomers by enzymes. These monomers enter the cytosol of the cell During glycolysis 2 ATP and 2 NADH are produced Pyruvate enters the mitochondria and is converted to a 2 carbon acetyl group which is attached to coenzyme A --> acetyl CoA Large amounts of acetyl CoA are also produced by the oxidation of fatty acids which are carried in the bloodstream, imported into cells and moved into mitochondria. The acetyl group is linked through a high-energy linkage and is easily transferable to other molecules. 1st it is transferred to oxaloacetate, a 4 carbon molecule. The acetyl group is oxidized to carbon dioxide in the citric acid cycle. Large amounts of the electron carrier NADH are generated. These electrons are then passed along an electrontransport chain within the mitochondrial inner membrane, where ATP is generated by oxidative phosporylation. About 106 ATP molecules are found in a typical cell, used up and replaced every 1-2 seconds!! About 50% of the free energy released is useful - the rest is lost as heat. This process will be covered more thoroughly in chapter 13. Glycolysis takes place in the cytosol of most cells, including many anaerobic microorganisms No oxygen required May have evolved early in the history of life, before photosynthetic organisms produced enough oxygen for oxidative phosphorylation. Each of the ten steps is catalyzed by a different enzyme (--ase) and produces a different sugar intermediate. Although no oxygen is involved, oxidation occurs, electrons are removed by NAD+ (producing NADH) from some of the carbons derived originally from glucose. Some of the energy released drives the direct synthesis of ATP. A net gain of two molecules of ATP and two NADH are produced. In aerobic organisms, electrons are then passed along the electrontransport chain to oxygen, forming water. Glucose is phosphorylated by ATP This step traps glucose inside the cell. Isomerization moves the carbonyl oxygen from carbon 1 to carbon 2 The entry of sugars into glycolysis is controlled at this step through regulation of the enzyme phosphofructokinase Cleaved to produce two three-carbon molecules. Steps 6 and 7 will be covered in Figure 4-5. In steps 6-10 ATP and NADH are generated along with pyruvate, a three carbon sugar. If oxygen is present, pyruvate and NADH enter the mitochondria in eukaryotes. In prokaryotes, cellular respiration takes place in the cytosol and cellular membrane. In anaerobic conditions, pyruvate and NADH stay in the cytosol rather than entering the mitochondria. Muscle cells when oxygen is limited. Remember, aerobic prokaryotes (bacteria) do not have mitochondria, but they do have cellular respiration = Kreb’s cycle and oxidative phosphorylation (electron-transport chain). 2 Fermentation generates NAD+, needed for step 6 of glycolosis. Without the generation of NAD+, glycolysis would be blocked at step 6. What intermediate would accumulate? This pathway was studied in yeast, in cell extracts, and has been understood for more than 50 years 2 Oxydation of an aldehyde to a carboxylic acid is coupled to the formation of ATP and NADH. Overall energetically favorable. These reactions are the only ones in glycolysis that create a high-energy phosphate linkage directly from inorganic phosphate. These reactions are the only ones in glycolysis that create a high-energy phosphate linkage directly from inorganic phosphate. • Getting an energetically unfavorable reaction to go – couple it to an energetically favorable reaction (total change in free-energy must be negative) • ATP ADP + P • ATP AMP + P-P AMP + 2 P (Figure 3.34) • activated intermediate use energy in the high energy bond to transfer a chemical group (Figure 3-27) • couple to a reaction that “uses up” the product of the 1st reaction to “pull the 1st reaction along” (Figure 3.22) Remember concentration of reactants and products affects the free-energy change of a reaction -11 to –13 kcal/mole Total = 26 kcal/mole • Getting an energetically unfavorable reaction to go – couple it to an energetically favorable reaction (total change in free-energy must be negative) • ATP ADP + P • ATP AMP + P-P AMP + 2 P (Figure 3.34) • activated intermediate use energy in the high energy bond to transfer a chemical group (Figure 3-27) • couple to a reaction that “uses up” the product of the 1st reaction to “pull the 1st reaction along” (Figure 3.22) Remember concentration of reactants and products affects the free-energy change of a reaction • Getting an energetically unfavorable reaction to go – couple it to an energetically favorable reaction (total change in free-energy must be negative) • ATP ADP + P • ATP AMP + P-P AMP + 2 P (Figure 3.34) • activated intermediate use energy in the high energy bond to transfer a chemical group (Figure 3-27) • couple to a reaction that “uses up” the product of the 1st reaction to “pull the 1st reaction along” (Figure 3.22) Remember concentration of reactants and products affects the free-energy change of a reaction • Arsenate (AsO43-) is chemically very similar to phosphate (PO43-) and is used as an alternative substrate by many phosphaterequiring enzymes. In contrast to phosphate, however, the high energy arsenate bond is quickly hydrolyzed in water, requiring no enzyme. Why is arsenate a compound of choice for murderers, but not for cells? Question 4-2. pyruvate In the presence of oxygen, pyruvate moves into the mitochondria and is decarboxylated to acetyl CoA. Fatty acids are also degrades to produce acetyl CoA In aerobic metabolism, the pyruvate produced by glycolysis is rapidly decarboxylated in the mitochondria by a giant complex of three enzymes called pyruvate dehydrogenase complex. CO2, NADH+ and acetyl CoA are produced Enzymatic breakdown (digestion) occurs in our intestine outside cells or in lysosomes inside cells Glycolysis = a chain of reactions which converts glucose (6 carbons) into 2 pyruvate (3 carbons) Large polymeric molecules are broken down into their monomers by enzymes. These monomers enter the cytosol of the cell During glycolysis 2 ATP and 2 NADH are produced Pyruvate enters the mitochondria and is converted to a 2 carbon acetyl group which is attached to coenzyme A --> acetyl CoA Large amounts of acetyl CoA are also produced by the oxidation of fatty acids which are carried in the bloodstream, imported into cells and moved into mitochondria. Lipid droplets in the cytoplasm are composed of triacylglycerols. These are cleaved into glycerol and three fatty acids. The majority of useful energy extracted from oxidation of food comes from pyruvate (glucose) and fatty acids. Enzymes in the mitochondria also degrade fatty acids, trimming 2 carbons at a time from its carboxyl end. Acetyl CoA, NADH and FADH2 are produced. Enzymes in the mitochondria also degrade fatty acids, trimming 2 carbons at a time from its carboxyl end. Acetyl CoA, NADH and FADH2 are produced. The majority of useful energy extracted from oxidation of food comes from pyruvate (glucose) and fatty acids. The citric acid cycle (tricarboxylic acid cycle) (Krebs cycle) accounts for about two-thirds of the total oxidation of carbon compounds in most cells. CO2 and high-energy electrons in NADH are the major products. These highenergy electrons are then passed to a membranebound electron-transport chain (oxidative phosphorylation) and finally accepted by O2 to produce water – H2O. NAD+ must be regenerated, therefore oxygen is required as a final electron acceptor to keep this cycle going. GTP is a close relative of ATP and transfer of its terminal phosphate group to ADP produces ATP FADH2 is another activated carrier molecule produced in the Krebs cycle. It is a carrier of highenergy electrons and hydrogen. Oxygen atoms required to produce CO2 are supplied by water. Three molecules of water are split each cycle. Oxygen here is from water. • The citric acid cycle also produces vital carbon-containing intermediates like oxaloaacetate, which are transferred back from the mitochondria into the cytosol where they serve as precursors for synthesis of many essential molecules, such as amino acids. The last step in the degradation of food molecules is oxidative phosphorylation or the electrontransport chain. The enzymes involved are specialized electron acceptor and donor molecules. These enzymes are embedded in the mytochondrial membrane. As the high energy electrons are passed from acceptor to donor, hydrogen protons are pumped across the membrane, setting up a large concentration/electric gradient. This gradient of H+ ions is used to generate ATP by the phosphorylation of ADP. Total oxidation of a molecule of glucose produces about 30 molecules of ATP. Starch and glycogen differ only in the frequency of branch points. Glycogen has many more branches than starch. Fat is far more important in energy storage than glycogen. Oxidation of fat releases 2xs as much energy and takes far less space since it doesn’t bind water. The average adult stores enough glycogen for only about a day, but has enough fat for nearly a month. During periods of light, photosynthetic cells convert some sugars made into starch and fats. Adipose Tissue = Fat cells Plants produce NADPH and ATP by photosynthesis in the chloroplast. However, most of the plants ATP needs are met by their mitochondria. Sugars are exported out of the chloroplasts into the mitochondria. During periods of light, photosynthetic cells convert some sugars made during photosynthesis into starch and fats. (see figure 4.15) Plant fats are triacylglycerols, but contain predominantely different fatty acids than animal cells (more unsaturated vs. saturated). Many biosynthetic pathways begin with glycolysis or the citric acid cycle. Many of the intermediates are siphoned off by other enzymes to produce amino acids, nucleotides, etc. The complexity of this network of metabolic pathways is matched only with the stringent controls placed at each branching. The metabolic balance of a cell is amazingly stable. The cell can adapt to starvation, damage, or disease – to a certain extent. The cell survives due to an elaborate network of controls, and redundant pathways.





