lightplus - Department of Physics & Astronomy
advertisement

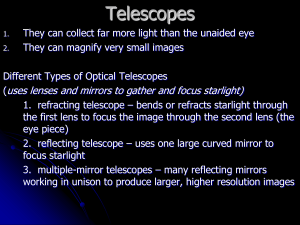
The Electromagnetic Spectrum, Light, Astronomical Tools Light and Other Forms of Radiation The Electromagnetic Spectrum In astronomy, we cannot perform experiments with our objects (stars, galaxies, …). The only way to investigate them is by analyzing the light (and other radiation) which we observe from them. Light as a Wave c = 300,000 km/s = 3*108 m/s • • Light waves are characterized by a wavelength and a frequency f. f and are related through f = c/ Light as a Wave • Wavelengths of light are measured in units of nanometers (nm) or angstrom (Å): 1 nm = 10-9 m 1 Å = 10-10 m = 0.1 nm Visible light has wavelengths between 4000 Å and 7000 Å (= 400 – 700 nm). The Electromagnetic Spectrum Wavelength Frequency Need satellites to observe High flying air planes or satellites Light as Particles • Light can also appear as particles, called photons (explains, e.g., photoelectric effect). • A photon has a specific energy E, proportional to the frequency f: E = h*f h = 6.626x10-34 J*s is the Planck constant. The energy of a photon does not depend on the intensity of the light!!! Temperature Scales • Want temperature scale with energy proportional to T – Celsius scale is “arbitrary” (Fahrenheit even more so) • 0o C = freezing point of water • 100o C = boiling point of water – By experiment, available energy = 0 at “Absolute Zero” = – 273oC (-459.7oF) – Define “Kelvin” scale with same step size as Celsius, but 0K = 273oC = Absolute Zero • Use Kelvin Scale for most astronomy work – Available energy is proportional to T, making equations simple (really! OK, simpler) – 273K = freezing point of water – 373K = boiling point of water – 300K approximately room temperature Planck “Black Body Radiation” • Hot objects glow (emit light) as seen in PREDATOR, etc. – Heat (and collisions) in material causes electrons to jump to high energy orbits, and as electrons drop back down, some of energy is emitted as light. • The hotter the material the more energy it emits as light – As you heat up a filament or branding iron, it glows brighter and brighter • The hotter the material the more readily it emits high energy (blue) photons – As you heat up a filament or branding iron, it first glows dull red, then bright red, then orange, then if you continue, yellow, and eventually blue Planck and other Formulae • Planck formula gives intensity of light at each wavelength – It is complicated. We’ll use two simpler formulae which can be derived from it. • Wien’s law tells us what wavelength has maximum intensity Max 3,000,000 nm K 3,000 m K T T • Stefan-Boltzmann law tells us total radiated energy per unit area E T 4 where 5.67 10 -8 J/(m 2 s K 4 ) From our text: Horizons, by Seeds Example of Wien’s law • What is wavelength at which you glow? – Room T = 300 K so Max 3,000 m K 3,000 m K 10 m T 300 K – This wavelength is about 20 times longer than what your eye can see. Thermal camera operates at 7-14 μm. • What is temperature of the sun – which has maximum intensity at roughly 0.5 m? 3,000 m K 3,000 m K T 6,000 K Max 0.5 m From our text: Horizons, by Seeds Kirchoff’s laws • Hot solids emit continuous spectra • Hot gasses try to do this, but can only emit discrete wavelengths • Cold gasses try to absorb these same discrete wavelengths Continuous Spectrum The spectrum of a common (incandescent) light bulb spans all visible wavelengths, without interruption. Emission Line Spectrum A thin or low-density cloud of gas emits light only at specific wavelengths that depend on its composition and temperature, producing a spectrum with bright emission lines. Absorption Line Spectrum A cloud of gas between us and a light bulb can absorb light of specific wavelengths, leaving dark absorption lines in the spectrum. Atomic (Hydrogen) Lines • • • Energy absorbed/emitted depends on upper and lower levels Higher energy levels are close together Above a certain energy, electron can escape (ionization) • Series of lines named for bottom level – To get absorption, lower level must be occupied • Depends upon temperature of atoms – To get emission, upper level must be occupied • Can get down-ward cascade through many levels n=3 n=2 n=1 From our text: Horizons, by Seeds Astronomical Telescopes Often very large to gather large amounts of light. In order to observe forms of radiation other than visible light, very different telescope designs are needed. The northern Gemini Telescope on Hawaii The Powers of a Telescope: Size does matter! 1. Light-gathering power: Depends on the surface area A of the primary lens / mirror, proportional to diameter squared: A = (D/2)2 D The Powers of a Telescope (II) 2. Resolving power: Wave nature of light => The telescope aperture produces fringe rings that set a limit to the resolution of the telescope. Astronomers can’t eliminate these diffraction fringes, but the larger a telescope is in diameter, the smaller the diffraction fringes are. Thus the larger the telescope, the better its resolving power. min = 1.22 (/D) For optical wavelengths, this gives min = 11.6 arcsec / D[cm] min Seeing Weather conditions and turbulence in the atmosphere set further limits to the quality of astronomical images Bad seeing Good seeing The Powers of a Telescope (III) 3. Magnifying Power = ability of the telescope to make the image appear bigger. A larger magnification does not improve the resolving power of the telescope! The Best Location for a Telescope Far away from civilization – to avoid light pollution The Best Location for a Telescope (II) Paranal Observatory (ESO), Chile http://en.wikipedia.org/wiki/Paranal_Observatory On high mountain-tops – to avoid atmospheric turbulence ( seeing) and other weather effects Adaptive Optics Computer-controlled mirror support adjusts the mirror surface (many times per second) to compensate for distortions by atmospheric turbulence The Spectrograph Using a prism (or a grating), light can be split up into different wavelengths (colors!) to produce a spectrum. Spectral lines in a spectrum tell us about the chemical composition and other properties of the observed object Radio Astronomy Recall: Radio waves of ~ 1 cm – 1 m also penetrate the Earth’s atmosphere and can be observed from the ground. Radio Telescopes Large dish focuses the energy of radio waves onto a small receiver (antenna) Amplified signals are stored in computers and converted into images, spectra, etc. Radio Interferometry Just as for optical telescopes, the resolving power of a radio telescope depends on the diameter of the objective lens or mirror min = 1.22 /D. For radio telescopes, this is a big problem: Radio waves are much longer than visible light Use interferometry to improve resolution! The Very Large Array (VLA): 27 dishes are combined to simulate a large dish of 36 km in diameter. The Largest Radio Telescopes The 100-m Green Bank Telescope in Green Bank, West Virginia. The 300-m telescope in Arecibo, Puerto Rico Infrared Telescopes Spitzer Space Telescope WIRO 2.3m NASA’s Great Observatories in Space (I) The Hubble Space Telescope • Launched in 1990; maintained and upgraded by several space shuttle service missions throughout the 1990s and early 2000’s • Avoids turbulence in Earth’s atmosphere • Extends imaging and spectroscopy to (invisible) infrared and ultraviolet NASA’s Great Observatories in Space (II) The Compton Gamma-Ray Observatory Operated from 1991 to 2000 Observation of high-energy gamma-ray emission, tracing the most violent processes in the universe. NASA’s Great Observatories in Space (III) The Chandra X-ray Telescope Launched in 1999 into a highly eccentric orbit that takes it 1/3 of the way to the moon! X-rays trace hot (million degrees), highly ionized gas in the universe. Two colliding galaxies, triggering a burst of star formation Very hot gas in a cluster of galaxies Saturn The Highest Tech Mirrors Ever! • Chandra is the first X-ray telescope to have image as sharp as optical telescopes. NASA’s Great Observatories in Space (IV) The Spitzer Space Telescope Launched in 2003 Infrared light traces warm dust in the universe. The detector needs to be cooled to -273 oC (-459 oF). Kepler’s Supernova with all three of NASA’s Great Observatories • Just 400 years ago: (Oct. 9, 1604) • Then a bright, naked eye object (no telescopes) • It’s still blowing up – now 14 light years wide and expanding at 4 million mph. • There’s material there at MANY temperatures, so many wavelengths are needed to understand it.