Chemical Equilibrium
advertisement

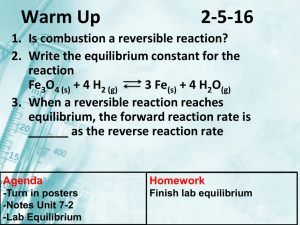
Chemical Equilibrium A StarLogo Tool for Chemistry Teachers Team Members Rey Martinez Janet Penevolpe Deborah Haggerton Jason Goldberg (here in spirit) The Problem How to teach equilibrium with less confusion. The Problem of Equilibrium First there must be a reversible reaction Then the “equal” part is the forward reaction rate and the reverse reaction rate The Problem expanded Le’ Chatelier’s principle states that a system in equilibrium that is disturbed will counteract that disturbance and establish a new equilibrium. Literature Research The concept of equilibrium is well documented. A plethora of full-text articles were found in which chemical equilibrium was of primary focus or in which Le Chatelier's Principle was an essential part. Literature Research Le Chatelier's Principle states that, if a closed system at equilibrium is subjected to change, processes will occur that tend to counteract that change. Literature Research According to Sprague, Trey, Pillay & Khan (2005), Le Chatelier’s Principle remains to be the most difficult concept for high school students to comprehend. Literature Research It has been found that students are able to make observations of chemical processes at the macroscopic level (e.g., color change) but tend not to be able to explain why these changes occur at the molecular level. Literature Research An elaborate study by Azizoglu, Alkan & Geban (2006), revealed that high school students may be confused with the concepts because their instructors do not have an adequate understanding. Literature Research According to the authors, the two most misunderstood concepts in chemistry are Le Chatelier's principle and the ideal gas law Literature Research Hanson, from St. Olaf College, suggests the use of playing-cards to explore statistical aspects of equilibrium (2003) While others have posted Web pages featuring conventional definitions, digital images and practical examples using common household items. Literature Research This finding is alarming. Consequently, the idea to create a computer simulation that offers students an opportunity to understand unobservable phenomena is the focus of the project. Literature Research To our knowledge, there are no other agent-based models of Le Chatelier’s principle in existence. Procedure Questions for programming How to model the general reaction ? A+BC How do the reactants disappear? How to get the product to breakdown? Procedure Agent - Agent interactions Reactant A responds to B Reactant B responds to A Product C has no response Procedure Agent - Environment interactions There are none Now here’s our model eqdeb05.slogo Experiments to analyze output data or use as Demonstrations Can you find equilibrium: with only reactants? with only products? faster with double reactants or products? Experiments to analyze output data or use as Demonstrations Le Chatelier What happens to equilibrium when: Add A or Subtract A? Add B or Subtract B? Add C or Subtract C? Add A & B or Subtract A & B? Add A & B & C or Subtract all? Citations Azizoglu N., Alkan M., & Geban O. (2006). Undergraduate pre-service teachers' understandings and misconceptions of phase equilibrium. Journal of Chemical Education, vol. 83, issue 6, pp. 947-959. Erickson, F. (1998). Qualitative methods for science education. In B.J. Fraser & K.G. Tobin (ed), International Handbook of Science Education (pp. 1155-1173). Dordretch: Kluwer Academic Publishers. Citations continued Hanson, R.M. (2003). Playing-card equilibrium. Journal of Chemical Education. vol.80, issue 11, pg. 1271. http://www.chemguide.co.uk/physical/equil ibria/introduction.html#top http://www.chm.davidson.edu/java/LeChat elier/LeChatelier.html