GEOG101L_Lesson_13-14

Office Hours
Tue: 12:30 PM to 2:30 PM
Wed: 9:00 AM to 10:30 AM & 12:00 PM to 2:00 PM
Thr: 9:00 AM to 10:30 AM
Course Syllabus can be found at: http://www.wx4sno.com/portfolio/BSU/Fall_2011/
This lecture will be posted AFTER class at: http://www.wx4sno.com/portfolio/BSU/Fall_2011/Lectures/
Lesson 13
Air Pressure
Hess, McKnight’s Physical Geography, 10 ed.
97-103 pp.
Air Pressure
Air pressure is the force exerted by the atmosphere along a surface
◦ Can be at ground level
◦ Or can be at any height above ground level
(AGL)
Air pressure is caused by atmospheric gases being pulled toward Earth by gravity
◦ Force can be measured, usually in either inches of mercury or millibars
Air Pressure, cont.
Factors Influencing Air Pressure
Pressure
Density
Temperature
All three are related
◦ If one changes, the other two change as well
The following are generalizations not laws pertaining to air pressure
Air Pressure Generalizations
Rising air produces low pressure near a surface
◦ Strong rising air can lead to the development of dynamic lows
Descending or “subsiding” air produces high pressure near a surface
◦ Strong subsidence results in dynamic highs
Warm temperatures produce low pressure near a surface
◦ Known as thermal lows
Cold temperatures produce high pressure near a surface
◦ Known as thermal highs
Dynamic High & Low Pressure
Descending air leads to surface high pressure
Rising air leads to surface low pressure
Thermal High & Low Pressure
High
Low
Warm air rises, which produces low pressure at a surface
Cold air sinks, produces high pressure at a surface
Measuring Air Pressure
Two systems of measurement:
◦ Inches of mercury (inHg)
Height of mercury within a vacuum column
Sea level: 29.92 inHg
◦ Millibars (mb)
Measure of force pressing down on a surface
1mb = 1000 dynes cm -2
1 dyne = force required to accelerate 1 gm -2 1 cm -2
Sea level: 1013.25 mb
◦ The larger the number, the higher the pressure
Isobars
Differences in pressure can be mapped with lines of equal pressure, known as isobars
Elongated areas of high pressure are known as ridges
Elongated areas of low pressure are known as troughs
Isobar Map
Station models
Weather observing stations (humanoperated and automatic) are located around the world
These stations report temperature, pressure, and a lot more information about the current weather
Station models, cont.
For this lesson, we’re only concerned with temperature and pressure
Temperature is located in upper-left
◦ Always measured in degrees Fahrenheit
Pressure is located on upper-right
◦ Given as an abbreviated measurement…needs to be converted…
Station models, cont.
To read the correct pressure:
◦ Add either a “9” or a “10” in front depending on which would bring the value closer to 1000.0.
◦ Then add a decimal before the very last digit
In the example above, 998 is given on the station plot
◦ Adding a “9” in front and a decimal before the last digit give us
999.8 mb
Pressures generally fall between 950.0 mb and 1050.0 mb
Station Model Pressure Examples
986.5 mb
1013.8 mb
Drawing Isobars
Connect station plots with equal pressure values
Some isolines will fall between stations
Lesson 14
Humidity
Hess, McKnight’s Physical Geography, 10 ed.
134-138 pp. & A8, A-9
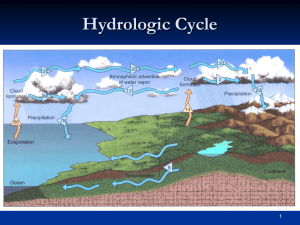
Humidity
Humidity is the amount of water vapor in a sample of air
Two important ways to measure it for this lab
◦ Mixing ratio (g/kg)
◦ Relative humidity (%)
Mixing Ratio
Mixing ratio is the actual amount of water vapor in a sample of air
◦ Grams water vapor per kilogram dry air (g/kg)
Mixing ratio does not change as the volume of air changes
The greatest amount of water vapor a parcel of air can hold is known as the saturation mixing ratio
◦ At this point the air is completely saturated and condensation occurs
Recall, saturation is when there is 100% water vapor in air and when that happens, water vapor goes from a gas to a liquid through condensation
Relative Humidity
A comparison between actual amount of water vapor in the air to the maximum amount of water vapor the air can hold at a given temperature
◦ This is also known as capacity
𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝐻𝑢𝑚𝑖𝑑𝑖𝑡𝑦 𝑅𝐻 =
𝐴𝑐𝑡𝑢𝑎𝑙 𝑤𝑎𝑡𝑒𝑟 𝑣𝑎𝑝𝑜𝑟 𝑐𝑜𝑛𝑡𝑒𝑛𝑡
𝑊𝑎𝑡𝑒𝑟 𝑣𝑎𝑝𝑜𝑟 𝑐𝑎𝑝𝑎𝑐𝑖𝑡𝑦
Expresses the amount of saturation in a percentage (%)
Calculating Relative Humidity
𝑀𝑖𝑥𝑖𝑛𝑔 𝑅𝑎𝑡𝑖𝑜
𝑅𝐻 =
𝑆𝑎𝑡𝑢𝑟𝑎𝑡𝑖𝑜𝑛 𝑀𝑖𝑥𝑖𝑛𝑔 𝑅𝑎𝑡𝑖𝑜 𝑥 100
For example, if we have a mixing ratio of 13.5 g/kg and a saturation mixing ratio of 22.5 g/kg, relative humidity would be:
𝑅𝐻 =
13.5 𝑔/𝑘𝑔
22.5 𝑔/𝑘𝑔 𝑥 100 = 60%
Things to Consider
As temperature increases, water vapor capacity also increases
◦ This means that as temperature increases, relative humidity decreases
As temperature decreases, water vapor capacity decreases
◦ As temperature decreases, relative humidity increases
Dew Point
Temperature at which relative humidity is 100%
Water vapor content = water vapor capacity
Dew point temperature can never be higher than actual air temperature
◦ When air temperature = dew point temperature, condensation occurs
Sling Psychrometer
Two thermometers mounted side-by-side
The bulb of one thermometer is exposed to the air, like a normal thermometer
◦ Dry-bulb thermometer
The bulb of the second thermometer is wrapped in cloth soaked in distilled water
◦ Wet-bulb thermometer
See page 74 for more information
Finally…
Omit problem 3d on page 77
Only do Part 1 & 2 problems (S.I.
Units)…omit pages 79 and 80.
◦ You can do pages 79 and 80 for up to 1 point extra credit