Chapter_4 - Weather Underground
advertisement

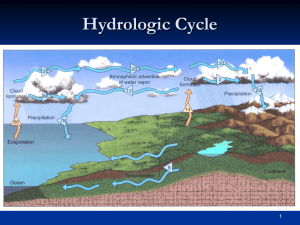
Chapter 4: Humidity, Condensation and Clouds By the end of this chapter you should: Know the hydrologic cycle Understand the idea of saturation Grasp the idea of humidity Understand the different cloud types, including fog Fig. 4-1, p. 80 Circulation of Water in the Atmosphere Evaporation from oceans that take up 70% of the Earth’s surface (solar radiation does the evaporation) Precipitation clouds produce rain on land (runs back to the ocean) and over the ocean Condensation this evaporated water (water vapor) turns back into liquid water in the form of clouds Evaporation, Condensation and Saturation What is equilibrium? It is a state in a system where competing forces are balanced For example, look at the two beakers below Evaporation, Condensation and Saturation In the first beaker is water with molecules bumping, vibrating at different speeds (temperature) Occasionally, one of the fast-moving molecules near the top of the water will break away and evaporate Evaporation, Condensation and Saturation At the same time, some of the water vapor molecules outside the water change phase from vapor to liquid Now let’s look at the second beaker Evaporation, Condensation and Saturation The second beaker has a lid on it Water molecules still evaporate into the air, and vapor still condenses into liquid But, what will happen if we let this process go on for a long time (think of a soda can in the sun) Evaporation, Condensation and Saturation Eventually, the number of water molecules changing phase into vapor will equal the number of vapor molecules changing phase into water This is called saturation. Basically, the air is in equilibrium Evaporation, Condensation and Saturation If we remove the lid and blow, water vapor molecules would go away. The air isn’t saturated anymore. This allows for more evaporation Thus, wind enhances evaporation Evaporation, Condensation and Saturation In the air above the beakers is Nitrogen, Oxygen, etc. But also dirt, smoke, salt, etc. Condensation nuclei – particles that allow vapor to condense into liquid Condensation is more likely when the air is cooled Vapor Pressure In a parcel of air, the pressure is 1000 millibars Pressure is the sum of the forces of every molecule pushing against the side of the parcel Vapor Pressure Thus, since air is 78% Nitrogen, the Nitrogen air pressure is 780 mb (1000 * 78%) Oxygen’s (21%) pressure is 210 mb Water vapor’s (1%) pressure is 10 mb. This is called actual vapor pressure Vapor Pressure So actual vapor pressure is a small portion of the total pressure Actual vapor pressure is an indication of how much water vapor is in the air Vapor Pressure If the air inside the balloon was saturated, the water molecules would exert a pressure. This is called saturation vapor pressure Vapor Pressure So, if we increase the temperature in the second beaker, more water molecules will escape to vapor To maintain equilibrium, the air must be able to hold more vapor At higher temperatures, it takes more vapor to saturated the air Fig. 4-5, p. 83 Relative Humidity Relative humidity – basically tells us how close the air is to being saturated Definition: RH = water vapor content / water vapor capacity OR RH = act vapor pressure / sat vapor pressure So an RH of 50% tells us what? Or 100% How about 104% • When the general public uses the term “humidity”, they mean “relative humidity.” Relative Humidity Supersaturation – air with a relative humidity greater than 100% Name two ways we can change the humidity Change the water vapor content (put more vapor in or take some out) Changing the air temperature Relative Humidity Higher temperatures – faster moving molecules and less likely they are to collide and condense Condensation occurs at saturation So what happens to RH during the day typically? Fig. 4-7, p. 85 Relative Humidity and Dew Point Suppose the morning temperature is 10°C and the RH is 100%. From graph, saturation vapor pressure is 12 mb at 10°C. What is the actual vapor pressure? Suppose the afternoon temperature is 30°C with no change is vapor content. What is the RH? Answer 1: 12 mb Answer 2: 29% (saturation vapor pressure is 412 mb at 30°C) For the second scenario, what temperature do we have to cool the temperature to to get saturation? Answer: 10°C For this scenario, 10°C is the dew point Relative Humidity and Dew Point Dew point – the temperature to which the air would have to be cooled to obtain saturation (with no moisture content change) So, temp of 15°C and RH of 100%. What is the dew point? High dew points represent a lot of moisture. Low dew points, low moisture Relative Humidity and Dew Point Relative Humidity and Dew Point Relative Humidity and Human Discomfort So why do humid days “feeling” hotter than drier days? First, why do we perspire? The amount of evaporation from the skin is directly related to how saturated the air is Drier air will evaporate sweat more quickly than air that is closer to saturation More evaporation, more cooling • “It’s not the heat, it’s the humidity” Relative Humidity and Human Discomfort Heat index – combines temperature and relative humidity to determine what the air “feels like” Dew and Frost On calm, clear nights, surface cools rapidly by what process? Air near the ground cools to dew point quickly, reaches saturation Dew - vapor that condenses on blades of grass Dew and Frost If the air temperature cools to the freezing point, you get frozen dew Frost - If the dew point is below the freezing point, then vapor goes directly to ice (what is this called)? Fog Fog – a cloud resting near the ground Types of fog: Radiation fog Advection fog Upslope fog Evaporation fog Fog Radiation fog – is produced as a result of radiational cooling Forms best at night with a shallow moist layer below a drier layer Moist layer cools rapidly and reaches dew point Usually “burns off” by afternoon from bottom up Radiation Fog Fog Advection fog – produced with warm, moist air flows over cool surface Very common along West Coast Cool water very near coast, warm water and moist air farther out. Afternoon breeze pushes air to coast, and cools it to dew point Moisture in fog important to redwood coast Advection Fog Advection Fog Advection Fog Fog Upslope fog – produced as moist air flows up an elevated surface As the air rises, it cools and reaches its dew point Common on the eastern slopes of the Rocky Mountains Can last for days Upslope Fog Fog Evaporation fog – produced by mixing two unsaturated air masses Breathing on a cold day produces a cloud Occurs above swimming pools If cold air moves over a swimming pool, warm water will evaporate into the air, raising the dew point Foggy Weather What areas will be the foggiest? Coastal or Interior? Where the water is!!!! The coast! Table 4-2, p. 98 High Clouds Cirrus High clouds made of ice crystals Can look like a pulled apart cotton ball High Clouds Cirrocumulus (mackerel sky) Made of ice crystals Looks a little like the scales of a fish “Cumulus” means has a puffy look High Clouds Cirrostratus Made of ice crystals “Status” means layered across entire sky Middle Clouds Altocumulus Mid-level clouds mostly made of liquid water “Cumulus” means puffy Can cause very pretty sunsets or sunrises Middle Clouds Altostratus Mid-level clouds mostly made of liquid water “Stratus” means layered across sky Low Clouds Nimbostratus Low cloud “Nimbo” or “Nimbus” means rain “Stratus” means layered across the entire sky Low Clouds Stratocumulus Most common cloud type in the world “Cumulus” means puffy, “Strato” means layered Clouds With Vertical Development Cumulus “Cumulus” means puffy Not all cumulus clouds grow into thunderstorms Clouds With Vertical Development Cumulus congestus “Cumulus” means puffy “Congestus” means bunched together Clouds With Vertical Development Cumulonimbus These are the big thunderstorms “Nimbus” means rain Some Unusual Clouds Lenticular clouds Pileus Mammatus clouds Contrails Nacreous Noctilucent • Several alleged ‘flying saucer’ reports have turned out to be lenticular clouds. Mammatus Clouds Lenticular Clouds Contrails Noctilucent Clouds Nacreous Clouds