First + Second week
advertisement

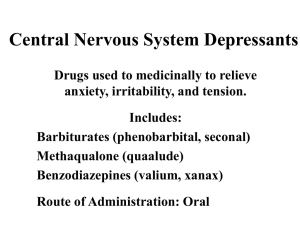
Components of PHL419 part II: • Toxcicity of Commonly Used Drugs ( CNS-acting drugs & cardiac glycosides). • Environmental Toxicity (Heavy metals , Pesticides & gaseous air pollutants) References - Casarett & Doull’s Toxicolology . The Basic Science of Poisons. 7th edition, 2008 by Curtis Klaassen - Classroom materials and lectures. 1. CNS-acting drugs - Most of CNS- acting drugs have potential risk of toxicity due to chronic uses, accedintal overdosage and /or drug misuse or abuse. - Most of CNS-acting drugs carry the risk of happening of tolerance, drug dependence and withdrawal syndrome once abruptly stop. - Tolerance: means decrease responsiveness to a drug following repeated exposure. This needs escalation of the drug dose to maintain the desired effect. - Drug dependence: The consequence of prolonged use of drugs that lead to recipient's dependence on the drug in both psychologic and physiologic (or physical) terms. Psychologic dependence includes neurotic behavior pattern of relapsing drug use. Users lose their control on their feeling of craving or tendency for the drug use (addiction). Physiologic (or physical) dependence can be described as an altered physiologic state that require continuous drug administration to prevent happening of withdrawal or abstinence syndrome. Withdrawal syndrome: are signs of physical debility and illness associated with abrupt stopping of drug use. Each CNS-acting drug has its own spectrum of withdrawal symptoms, but in most cases it will be the reverse of physiological effects. - For examples withdrawal symptoms of most of CNS depressant drugs involve signs of CNS excitability (increased anxiety, insomnia, tremors that may progress to convulsion). CNS-acting drugs include the following categories: I. Anixiolytic and hypnotic drugs: e.g. Barbiturates and benzdizipines II. Opiates and narcotic analgesics: e.g. Morphine, Codiene, Percoset, Percodan ….etc III. Drugs used to treat psychological disorders e.g. Antipsychotics (antischizophrenic drugs), and Antidepressants. IV. Stimulants: e.g. Cocaine, Amphetamines and Methamphetamines V. Hallucinogens and psychedelics ( recreational drugs or drugs of abuse e.g. Cannabis , LSD and Mescaline Drug Scheduelling Drugs are classified into one of five lists (schedules) based on the substance's medical utility, potential for abuse, and dependence liability. SCHEDULE I: • Drug has no current accepted medical use and has a high potential for abuse. • • Class examples: Heroin, Cannabis. LSD, GHB, Cathinone, marijuana and Pharmacies do not sell Schedule I drugs, and they are not available with a prescription by physician. SCHEDULE II: • • • • Drug has current accepted medical use and has high potential for abuse. Class examples: Cocaine, Morphine, Fentanyl, Codeine, methadone, meperidine, Amphetamine, Methamphetamines, amobarbital, secobarbital and pentobarbital. Schedule II drugs may be available with a prescription by a physician without refill, but not all pharmacies may carry them. These drugs require more stringent records and storage procedures than drugs in Schedules III and IV. SCHEDULE III • • • Drug has current accepted medical use and has moderately high potential for abuse. Class examples: Anabolic steroids, Ketamine, Hydrocodone with Aspirin, and Hydrocodone with Acetaminophen (Lortap). Schedule III drugs may be available with a prescription , most of pharmacies may carry them upon written or phone prescriptions with or without refill. SCHEDULE IV • • • Drugs have current accepted medical use and have moderate potential for abuse. Class examples: Chloral, benzodiazepines, meprobamates and Phenobarbital. These drugs may be available with a prescription with refill, all pharmacies may carry them. SCHEDULE V: • • Drug has accepted medical use and has lowest potential for abuse. Class examples: Lomotil (diphenoxylate and atropne), dextromethorphane and cough suppressants with codeine like Robitussin. Schedule V drugs are regulated but generally prescription is unnecessary. Schedule Schedule I Abuse Liability High Approved Medical Use No Availability investigational use only Examples Schedule II High Yes written prescription with no refills Schedule III Moderately High Yes written or telephone prescription with refills Schedule IV Moderate Yes written or telephone prescription with refills Schedule V Low Yes prescription not necessary marijuana, THC LSD, mescaline, peyote heroin amphetamine, methamphetamine, cocaine codeine, levorphanol, meperidine methadone, morphine, opium amobarbital, pentobarbital, secobarbital phencyclidine Tylenol with codeine, paregoric chlorphentermine anabolic steroids chloral hydrate chlordiazepoxide, diazepam, flunitrazepam meprobamate methohexital, phenobarbital Robitussin A-C (contains less than 100 mg codeine per 100 ml) Toxicity of Barbiturates • Barbiturates are a group of CNS depressants, class of drugs known as anixiolytics and hypnotics which generally describes their sleepinducing and anxiety-decreasing effects. • Barbiturates are big family (of over 2500 members), all are derived from barbituric acid, of which about 50 have been marketed. • • • Barbiturates act by potentiate the ability of the inhibitory neurotransmitter, GABA, to activate its specific type of receptors known as GABAA receptors. Barbiturates increase the effectiveness of GABA by altering the receptor so that GABA can bind more easily, an effect known as allosteric activation. Barbiturates in large doses, may be agonists at GABAA receptors, thus barbiturates produce potent CNS depressant effect. • Barbiturates are now little used as anxiolytic and hypnotic agents due to the narrow therapeutic margin and excessive side effects (sometime lethal). • Barbiturates are also addictive, produce physical dependence, and can cause a life-threatening withdrawal syndrome upon abrupt discontinuation of therapy. • Barbiturates also induce a high degree of tolerance that they strongly induce the synthesis of hepatic CYP450 and conjugating enzymes, and thus increase the rate of metabolic degradation of its own and many other drugs, giving rise to a number of potentially troublesome drug interactions. • Barbiturate toxicity: - Barbiturates users usually suffer from depressant side effects like drowsiness, sluggishness, some staggers, slurred speech, ataxia, nystagmus, headache and impaired concentration. - These CNS depressant effects of barbiturates may intensify with uses of other CNS depressants like opiates or ethanol (alcoholic beverages). - Barbiturates hangover: Hypnotic doses of barbiturates produce a feeling of tiredness and lack of concentration well after the patient wakes. This drug hangover may lead to impaired ability to function normally for many hours after waking. Occasionally, nausea and dizziness occur. Patients under barbiturate therapy are contraindicated to drive or operate dangerous machinery. - Physical dependence: Abrupt withdrawal from barbiturates therapy may cause abstinence symptoms that include tremors, anxiety, weakness, restlessness, nausea and vomiting, seizures, delirium, and cardiac arrest. Withdrawal is much more severe than other CNS depressants and can result in death. - Precautions: As noted previously, barbiturates induce the liver P450 system and, therefore, may decrease the duration of action and therapeutic efficacy of drugs that are metabolized by these hepatic enzymes (oral contraceptives, hypoglycemic drugs, anticoagulants,……etc) - barbiturates are also dangerous to patients suffering from the metabolic disease porphyria . - Poisoning: Barbiturate poisoning has been a leading cause of death resulting from drug overdoses due to severe depression of respirator and CV centers in brain stem. - This results in a shock-like condition with shallow, infrequent breathing. - Treatment includes only symptomatic interventions (no specific barbiturate antagonist is available) which include artificial respiration and purging the stomach of its contents if the drug has been recently taken. Hemodialysis may be necessary if large quantities have been taken. Alkalinization of the urine often aids in the elimination of barbiturates . Administration of pharmacologic antidote like respiratory stimulants like doxapram. Toxicity of Benzodiazepines • • • Benzodiazepines are the most widely used anxiolytic-hypnotic drugs. They have largely replaced barbiturates and meprobamate in the treatment of anxiety disorders and insomnia, because they are considered safer (high TI) and more effective. The basic chemical structure of benzodiazepines consists of a sevenmembered ring fused to an aromatic ring, with four main substituent groups that can be modified without loss of activity. Thousands of compounds have been made and tested, and about 20 are available for clinical use Like barbiturates, the targets for benzodiazepine actions are the GABAA receptors . Benzodiazepines enhance the response to GABA by facilitating the opening of GABA-activated chloride channels. They bind specifically to a regulatory site of the receptor, distinct from the GABA- binding site (and from barbiturates site), and act allosterically to increase the affinity of GABA for the receptor. • Major uses of benzodiazepines include: - Anixiolytics (reduction of anxiety and aggression ) e.g. Clonazepam, Lorazepam, Alprazolam and Diazepam - Hypnotics (induce sedation and induction of sleep leading to improvement of symptoms of insomnia ) e.g. Flurazepam, Temazepam, and Triazolam - Muscle relaxant (induce muscle relaxation through central mechanism totally not related to its anixiolytic–hypnotic action, spasmolytics, and excessivly used in treatment of muscle spasm like that happen with muscle strain, and in treating spasticity from degenerative disorders, such as multiple sclerosis and cerebral palsy. e.g. Diazepam - Anticonvulsant (suppression of convulsions (antiepileptic effect) used the treatment of epilepsy. e.g. Clonazepam, Diazepam, and Lorazepam. - Induce amnesia: Benzodiazepines obliterate memory of events experienced while under their influence .They utilized as premedication for minor surgical procedures such as endoscopic, bronchoscopic, and certain dental procedures can thus be performed without leaving unpleasant memories. They also cause a form of conscious sedation, allowing the person to be receptive to instructions during these procedures. e.g. Midazolam Benzodiazepines toxicity: • The main side effects associated with benzodiazepines therapy are drowsiness, confusion, amnesia and impaired coordination (ataxia), which considerably affects job performance daily skills such as driving. • Cognitive impairment (decreased long-term recall and acquisition of new knowledge) can also occur with use of benzodiazepines. • - - Tolerance is less marked with benzodiazepines with some exceptions, Triazolam, one of the most potent oral benzodiazepines with the most rapid elimination, often shows a rapid development of tolerance, early morning insomnia, and daytime anxiety, along with amnesia and confusion. Benzodiazepines are relatively safe in overdose than other anxiolytic/hypnotic drugs. In overdose, benzodiazepines cause prolonged sleep, without serious depression of respiration or cardiovascular function. Alcohol and other CNS depressants (like barbiturates, phenytoins, phenothizines,..etc) enhance the sedative-hypnotic effects of the benzodiazepines and may cause severe, life-threatening, respiratory depression upon overdosage. Benzodiazepines should be avoided in patients with liver disease and acute narrow-angle glaucoma (benzodiazepines increase IOP). - Flumazenil is a specific benzodiazepines antagonist . It is used as an antidote in the treatment of benzodiazepine overdose. - It reverses the effects of benzodiazepines by competitive inhibition at the benzodiazepine binding site on the GABAA receptor benzodiazepines. - The drug is available for IV administration only. Onset is rapid but duration is short, with a half-life of about 1 hour. Frequent administration may be necessary to maintain reversal of a long-acting benzodiazepine. Toxicity of Opiates • • • • Opiates, sometimes referred to as narcotic analgesics, are a group of drugs which are used medically as strong pain killers to relieve moderate to severe types of pains which exert their effects mainly through central actions. Some opiates originally isolated from a resin taken from the seeded pod (poppy capsule) of hemp plant (Papaver somniferum), while other opiates are semisynthesized or chemically manufactured (opoids). Opiates exert their actions through binding to different types of opiate receptors (μ, δ, and κ), which are found principally in the central nervous system and some peripheral areas (e.g. GIT and immune cells). Opiate receptors are a group of Gi-protein coupled receptors with endogenous opioids as ligands. The other opiates act as agonists with different affinity and efficacy to these receptors. • • Pure agonists: These opoids have high affinity for receptor binding plus high efficacy used in management of sever pains. They all have high affinity for μ receptors and generally lower affinity for δ and κ sites. They always cause both physical and psychological dependence. e.g. Morphine, methadone, hydromorphone, fentanyl, oxymorphone, and heroin. Weak aagonists: These drugs have high affinity for receptor binding but low efficacy. They are used mainly in management of moderate pain in addition to other therapeutic uses . both analgesic and unwanted effects, are less than those of morphine, and they have lower tendency to cause dependence. e.g. Propoxyphene, codeine, hydrocodone, oxycodone, dihydrocodone, methadone, dexropropoxyphene and pethedine. • • Partial agonists and mixed agonist-antagonists: These drugs may produce agonist effects at some opiate receptors and antagonist effects at another opiate receptors e.g Pentazocine, Nalbuphine, Nalorphine, and Dezocine. Nalbuphine is agonist on -receptor and potent anatagonist at receptor but weak antagonist at -receptors. Pentazocine is antagonist at μ-receptors but partial agonists on - and -receptors. Full Antagonists: These opiate drugs have affinity for binding with opiate receptors but no efficacy and block action of both endogenous and exogenous opiates. e.g. naloxone, nalmefene and naltrexone. - In addition to their strong analgesic effects they also have many other effects like cough supression and antidiarrheal effects. - Opiates are strong CNS depressant and produce narcosis (tranquil, sedating and euphoric effect). Both physical and psychological dependence are probable with continued use of opiates (addictive and strong tolerance). - Opiates have many legal medicinal uses in addition to high potential of abuses. - Members of opiates family are listed in different drug schedules. Therapeutic uses of Opiates : i. Pain management: - Relief of moderate to severe acute pain (Like postoperative pain, pain associated with orthopedic manipulations, myocardial infarction pain, cancer pain, renal colic) - To induce brief tranqulling effect with analgesia in serious and frightening conditions accompanied by pain (e.g. multiple traumas ) ii. Preanesthetic medication to reduce pain sensation and anxiety ( e.g Fentany, Pethidine) iii. Cough Suppression (e.g. codeine, dextromethorphan) iv. Symptomatic treatment of sever diarrhea and dysentery (e.g. Diphenoxylate, Loperamide) Narcotic Drug Most Common Uses Narcotic Schedule Heroin Abuse Heroin I Morphine Analgesia Morphine II,III Methadone Methadone II Meperidine Treat narcotic dependence Analgesia Fentanyl II Oxycodone Analgesia Hydromorphone II Propoxyphene Analgesia Meperidine II Codeine Analgesia, antitussive Codeine and its combinations II, III, V Loperamide Antidiarrheal Pentazocine IV Diphenoxylate Antidiarrheal Propoxyphene IV Opium tincture Antidiarrheal Opiates toxicity: • The main side effects associated with use of opiates include: 1- Apathy, lethargy drowsiness, mood disturbance and coordination impairment. 2- Constricted eye pupils, nystagmus with photophobia 3- Slow Shallow breathing, and slow heart rate. 4- Difficulty in breathing and itching (histamine-releasing) 5- Constipation, nausea and GIT upset. 6- Opiates depress appetite and thirst. 7-Loss of libido and impotence in men; amenorrhea and infertility in women. 8- The body's tolerance to pain is increased that delays awareness of serious medical conditions (abusers). Most of CNS depressant side effects of opiates are enhanced by concurrent administration of many CNS depressants like alcohol, barbiturates, benzodiazepines, phenothiazines, MAOIs, and TCAs. - Administration of overdose of morphine or other opiates (acute toxicity) usually associated with the following symptoms: Loss of conciousness Cyanosis, Low pulse and bradycardia Pinpoint pupils Anurea and constipation Hypothermia Flaccid muscles Death may be a result from either respiratory depression or acute pulmonary edema or both. Treatment of acute opiate toxicity include - Ventilation (carbogen; 95% O2 + 5% CO2) - Administeration of opiate antagonist (e.g. naloxone or naltrexone). Tolerance and physical dependence are manifestations of opiates chronic toxicity. • Tolerance to opiates develops rapidly, accompanied by physical withdrawal syndrome. It may result in the need for an escalation of the dose to maintain symptomatic improvement. • The mechanism of tolerance may involve the inhibitory effect on endogenous opiates, and opiates’ receptor internalization, desensitization (uncopling from Gproteins or downregulation (decrease receptor density which would reduce the response to the normal dose of the drug) all have been proposed as potential mechanisms of tolerance development. • Tolerance to opiates is develops rapidly to Analgesia, Euphoria, Sedation and Nausea, while tolerance occurs slowly to other effects like itching, urinary retention, and respiratory depression. However, tolerance does not develop to constipation or miosis. - • Tolerance does not become clinically manifested until after 2-3 weeks of frequent exposure to ordinary therapeutic doses. • It develops most readily when large doses are given at short intervals. • Cross-tolerance is developed between opioids • Some strategies are now developed (still within pre-clinical research) trying to actively reduce/prevent opiate tolerance trying to make patients get much analgesic benefits by concurrent administration of: CaM KII inhibitor e.g. KN-93 CCK inhibitors e.g. Proglumide NMDA glutamate receptor antagonist e.g. d-methadone CYP 450 inhibitor e.g. Cimetidine Withdrawal symptoms appear 4-6 hours following the last dose. Symptoms include: - Sever bone aches and muscle pain (back and extremities), irritability and fearfulness - Hyperventilation (increased respiratory rate) - Dysphoria, depression and generalized weakness - Restlessness, and insomnia - Increased blood pressure and hyperglycemia - Diarrhea, abdominal pain, nausea and vomiting. - Pupillary dilation (mydriasis) - Hyperthermia, excessive sweating and yawing. - Lacrimation, runny nose and piloerection - Spontaneous tremors. - Alternating episoded of chilliness and flushing with kicking movements of the legs (goose bumps). • • • The intensity of these symptoms depends on how much of the drug was taken, how often and for how long. These symptoms are usually strongest 24 to 72 hours after onset and can persist for seven to 10 days. Successful treatment of narcotic drug withdrawal symptoms is based on the idea that it is best slowly withdraw (tapering off), and give the patient enough drugs to get rid of withdrawal symptoms without causing mental clouding. Treatment with medicines: (14-21 days) (1) Detoxification: Detoxification: Opiate dependence is satisfied by μreceptor agonists, weak, long-acting μ-receptor agonists such as methadone, bupereonrphine,and diamorphine may be used (2) Supportive treatment: Symptomatic treatment of withdrawal signs (irritability, GIT effects, sever aching,…… etc). Clonidine can help by reducing the intensity of withdrawal symptoms by 50 – 75%. Clonidine is especially effective at reducing anxiety, cramping, muscle aches, restlessness, sweating, tears, and runny nose. Acetaminophen, or ibuprofen could help in pain and hyperthermia, loperamide can help with diarrhea, and hydroxyzine may ease nausea. Plenty of fluids and rest are important. • • Psychological Treatment: this may take few month, it is considered more important step in treatment of psychologic dependence or addiction. Precautions: Opiates should be avoided to be used in patients with the following pathologic disorders: i. Decreased impaired respiratory functions e.g. emphysema, asthma and CPOD. ii. Biliary colic iii. Head injury (increase in ICP) iv. Reduced blood volume v. Hepatic and renal insufficiency vi. During pregnancy and labour Toxicity of Antidepressants Depression • • • • It is the most common mood and affective disorders that defined as “ A mood disturbance characterized by feelings of sadness, despair, and loss of interest or pleasure in activities. These feelings may be accompanied by somatic complaints, such as changes in appetite, sleep disturbances (insomnia or oversleeping), restlessness or lethargy, and decreased concentration. Thoughts of injuring one's self, death, or suicide may also occur”. Depression strikes 10% - 15% of adults population, affecting all racial, ethnic, age, and socioeconomic groups. It's twice as common in women as in men and is especially prevalent among adolescents. Many theories have been postulated to illustrate mechanisms of depression development, but the most acceptable one is the monoamine theory which states that “monoamine neurotransmitters (NE, 5-HT) function in the expression of mood”. Depression is caused by a functionally deficit monoaminergic transmission in certain sites of the CNS (like cerebral cortex, hippocampus and amygdla), while mania results from a functional excess. Antidepressants • • • • • • • Tricyclic antidepressants: e.g. amitriptylline (Tryptazol), clomipramine (Anafranil), imipramine (Tofranil) Tetracyclic antidepressants: e.g. Mianserin (Lumin) Selective serotonin reuptake inhibtors (SSRIs): e.g. citalopram (Celapram), fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Aropax), sertraline (Zoloft) Selective noradrenaline reuptake inhibitor (SNaRIs ): e.g. reboxetine (solvex), maprotiline (lodumil) Irreversible MAO A inhibitors: e.g. Phenelzine (Nardil), Tranylcypromine (Parnate) Reversible MAO A inhibitors: e.g (manirex) moclobemide (Clobemix), modobemide Atypical antidepressants: e.g. Venlafaxine (Effexor ), Duloxetine (Cymbalta), Trazodone (Trazorel) , Miratazapine (Remeron), and Bupropion (Zyban). Tricyclic antidepressants: The mechanism of action of tricyclic antidepressants is mainly related to their ability to inhibit the transmitter-uptake mechanism of monoaminergic neurons. Therapeutic uses: 1. Antidepressant 2. Treatment of neuropathic pain 3. In migraine prophylaxis 4. Treatment of nucternal enuresis 5. Antianxiety ( OCD, Phobias…etc) 6. ADHD Tricyclic antidepressants toxicity: I. II. III. IV. V. VI. VII. Drowsness, slurred speech, sedation and weight gain (H1 block); Postural hypotension (α-adrenoceptor block) Flushing, dry mouth, blurred vision, excessive perspiration mydriasis, increase IOP, urine retention and constipation (muscarinic block). TCAs are contraindicated to be used in patients with glaucoma, patients with urinary pathology ) occasionally seizures (Contraindicated to be used in patients with convulsive disorders ) Tachycardia, disturbances in cardiac conduction with risk of ventricular dysrhythmias, congestive heart failure, myocardial infarction, heart block, asystole. ECG changes (prolong QT interval, inversion of T wave or widened QRS), mainly due to decrease the sodium influx through the fast Na+-channels. TCAs are contraindicated in patients with heart diseases, especially those who have a history of conduction disorders and in patients with thyrotoxicosis or those use thyroid drugs Dangerous in acute overdose: Convulsion, confusion, decrease mental alertness, palpitation , respiratory depression, cardiac dysrhythmias, and coma. TCAs should be avoided to administered simultaneously with many drugs like: a. CNS depressants; alcohol, anaesthetics, Barbiturates, Opioids, Benzodiazepinesand b. Hypotensive drugs like guanethidine, and resirpine (reverse their action and blocking its transport into sympathetic nerve endings) c. Anticholinergic agents or antiparkinsons (e.g., atropine, hyoscine, levodopa), neuroleptics with an anticholinergic action (e.g. Chlorpromazine) , exaggerate symptoms of anticholinergic especially urine retention and paralytic ileus with hyperexcitation states or delirium may occur, as well as attacks of glaucoma. d. Anti-arrhythmic agents of the quinidine type. e. MAOIs: TCAs should not be administered for a period of at least 14 days after the discontinuation of treatment with MAO inhibitors .The same caution should also be observed when administering an MAO inhibitor after previous treatment with TCAs. To avoid happening of Hypertensive episodes with excitement and hyperactivity. f. The plasma concentration of Imipramine may increase when the drug is given concomitantly with hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine) and decrease by concomitant administration with hepatic enzyme inducers (e.g., barbiturates, phenytoin), and adjustment of the dosage of Imipramine may therefore be necessary. g. Avoid the use of preparations, such as decongestants and local anesthetics, that contain any sympathomimetic amine (e.g. epinephrine, norepinephrine), since it has been reported that tricyclic antidepressants can potentiate the cardiovascular effects of catecholamines. Selective serotonin reuptake inhibitors (SSRIs) SSRIs are believed to increase the extracellular level of 5-HT by inhibiting its reuptake into the presynaptic cell, increasing its level in the synaptic cleft available to bind to the 5-HT postsynaptic receptor. 1. 2. 3. 4. 5. Therapeutic uses: Most widely prescribed drugs in treatment of major depressive disorders Anorexigenic agents in treatment of obesity Management of panic attack Anixiolytic (OCD, phobias, GAD) Treatment of alcohol dependence Toxicity: • • • • • • SSRIs are considered to have fewer adverse (less non-specific mechanisms) effects and less severe in toxicity and overdosage than other antidepressants. SSRIs administration is associated with headache, sweating, anxiety and agitation, nausea, vomiting, diarrhea, aneroxia, weakness and fatigue, sexual dysfunction, weight loss, insomnia. Overdoses: may be associated with seizures (much less cardiotoxicity) SSRIs act as inhibitors of hepatic cytochrome P450 isozymes (e.g CYP1A2, CYP2C19) that inhibit the liver metabolic rate and increase the risk of toxicity with concurrently administered drugs like like warfarin, digoxin, propafenone, propranolol, amitriptyline , sumatriptan, diazepam , carbamazepine, clozapine, and cyclosporine. Combination of SSRIs and other antidepressants like TCAs and MAOIs should avoided to avoid happening of “serotonin syndrome” that include hyperthermia, muscle rigidity, sweating, shivering, dilated pupils, confusion, restlessness myoclonus (clonic muscle twitching), or loss of muscle coordination and changes in mental status and vital signs. Death may occur from cardiovascular collapse. Therefore, extended periods of washout for each drug class should occur prior to the administration of the other class of drugs. Management of serotonin syndrome could be attributed by symptomatic treatment using of antiseizure drugs (lorazepam), Muscle relaxants (balcofen) and 5-HT blocker (cyproheptadine) • • SSRIs should be used for treating depression with great caution in children and adolescent under age of 18 due to adverse effects, including excitement, insomnia and aggression in the first few weeks of treatment . In addition to increased suicidal ideation. SSRIs have the potential for causing mild physical dependence and abstinence syndrome after their abrupt withdrawal. Possible include headache, malaise and flu-like symptoms, agitation and irritability, nervousness, and changes in sleep pattern. Depletion of serotonin stores may stands behind these symptoms. Gradual withdrawal is preferred. Monoamine oxidase inhibitors(MAOIs) • • • • • • • MAOIs are used much less than other antidepressants because of their adverse effects and serious interactions with other drugs and foods. They are indicated as last line for treatment of major depression in patients who have not responded to other drugs. MAOIs act by inhibiting the activity of monoamine oxidase, thus preventing the breakdown of monoamine neurotransmitters and thereby increasing their availability. There are two isoforms of monoamine oxidase, MAO-A and MAO-B. MAO-A preferentially deaminates serotonin, melatonin, epinephrine and norepinephrine. MAO-B preferentially deaminates phenylethylamine. Dopamine is equally deaminated by both types. Some MAOIs inhibited monoamine oxidase irreversibly. (e.g. Phenelzine & Tranylcypromine ) that permanently t, deactivate MAO and the enzyme cannot function until it has been replaced by the body, which can take about two weeks. A few newer MAOIs (e.g. moclobemide & modobemide) are reversible In normal human subjects, MAOIs cause an immediate increase in motor activity, and euphoria and excitement develop over the course of a few days. Main side effects: postural hypotension (sympathetic block); atropine-like effects (as with TCA); weight gain; CNS stimulation, causing restlessness and insomnia. headache, anxiety, flushing of the skin, and cardiovascular complications including hypertension and tachycardia are the most common symptoms of acute overdosage toxicity. MAOIs are CYP 450 inhibitors and should be used with great caution with other drugs. For example: Pethidine, concurrent adminsteration with MAOIs cause severe hyperpyrexia, with restlessness, coma and hypotension. Due it abnormal pethidine metabolite is produced because of inhibition of demethylation. MAOIs are also liable to interact with food elements especially those riche in indirect sympathomimetic “tyramine” like cheese, beer, yeast or soy cotaining food and othe indirectly-acting sympathomimetics like ephedrine , amphetamine to cause hypertensive crisis or Cheese reaction. Symptoms include severe hypertension with severe throbbing headache, high body temperature, seizures and occasionally even to intracranial haemorrhage. Such reactions can occur up to 2 weeks after treatment is discontinued. Atypical Antidepressants Atypical antidepressants, are a lot with variable mechanism of actions. In general they are more specific with less toxicity. such as ; • • • • Venlafaxine: it is a potent inhibitor of serotonin reuptake and, at medium to higher doses, is an inhibitor of norepinephrine re-uptake. It is also a mild inhibitor of dopamine reuptake at high doses. The most common side effects of venlafaxine are nausea, headache, sexual dysfunction, dizziness, insomnia, somnolence , and constipation. Duloxetine: is another atypical antidepressant that inhibits serotonin and norepinephrine reuptake at all doses GI side effects are common with duloxetine, including nausea, dry mouth, and constipation and CNS side effects including Insomnia, dizziness, somnolence, and sweating are also seen. Sexual dysfunction also occurs along with the possible risk for an increase in either blood pressure or heart rate. Bupropion: acts as a weak dopamine and norepinephrine reuptake inhibitor to alleviate the symptoms of depression. Also, it assists in decreasing the craving and attenuating the withdrawal symptoms for nicotine in tobacco users trying to quit smoking. Side effects may include dry mouth, sweating, nervousness, tremor, a very low incidence of sexual dysfunction, and an increased risk for seizures at high doses. Mirtazapine: Enhances serotonin and norepinephrine neurotransmission via mechanisms related to its ability to block presynaptic α-2 receptors. Additionally, it may owe at least some of its antidepressant activity to its ability to block 5-HT2 receptors. It is a sedative because of its potent antihistaminic activity, but it does not cause the antimuscarinic side effects of TCAsor interfere with sexual functioning, as do the SSRIs. Increased appetite and weight gain frequently occur. Mirtazapine is markedly sedating, which may be used to advantage in depressed patients having difficulty sleeping.