Naming Binary Compounds - Madison Public Schools
advertisement

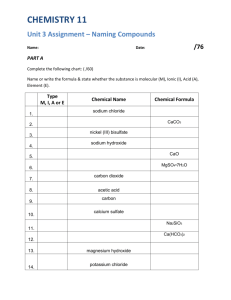
Section 4.1 Naming Binary Compounds What are the naming rules when couples marry? Section 4.1 Naming Binary Compounds Compounds Binary Ionic Polyatomic Ionic Acids Molecular Ammonium Phosphate LiC2H3O2 Nitrogen Trichloride Iron (III) Oxide CuI CuSO3 Sodium Carbonate Lead (II) Acetate Magnesium Sulfite CaBr2 I2Cl2 HNO3 Hydrochloric Acid Aluminum Hydroxide Ba3(PO4)2 Section 4.1 Naming Binary Compounds Compounds Binary Ionic Polyatomic Ionic Acids Ammonium Phosphate LiC2H3O2 Molecular Nitrogen Trichloride Iron (III) Oxide CuI CuSO3 Sodium Carbonate Lead (II) Acetate Magnesium Sulfite CaBr2 I2Cl2 HNO3 Hydrochloric Acid Aluminum Hydroxide Ba3(PO4)2 Section 4.1 Naming Binary Compounds Objectives 1. To learn to name binary compounds of a metal and nonmetal 2. To learn to name binary compounds containing only nonmetals 3. To summarize the naming of all types of binary compounds 1. AxBy where A is a metal, B is a non-metal 2. CxDy where C and D are non-metals Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal • Binary ionic compounds contain positive cations and negative anions. – Type I compounds • Metal present forms only one cation – Type II compounds • Metal present can form 2 or more cations with different charges Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal • For compounds containing both a metal and a nonmetal, the metal is always named first. The nonmetal is named from the root element name. Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal Rules A Type I Binary Ionic compounds Use your Machine to identify and name the following: NaCl, MgO, LiBr, CaF2, Al2O3 Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal Type II Binary Ionic compounds • Since the metal ion can have more than one charge, a Roman numeral is used to specify the charge. Rules B Gold (I) Chloride Gold (III) Chloride Section 4.1 Naming Binary Compounds A. Naming Compounds That Contain a Metal and a Nonmetal Type II Binary Ionic compounds Section 4.1 Naming Binary Compounds What are the Formulas that would be formed from the following ions and what are the Names of the compounds? Cu+ and FNi3+ and IFe2+ and O2Pd4+ and ClTi4+ and P3- Section 4.1 Naming Binary Compounds Name the following compounds: FeCl2 CoS CoBr3 PbI2 CuO SnO2 Fe2O3 Section 4.1 Naming Binary Compounds B. Naming Binary Compounds That Contain Only Nonmetals Type III Compounds – Non-metal/Non-metal Rules C Section 4.1 Naming Binary Compounds B. Naming Binary Compounds That Contain Only Nonmetals Type III Compounds Name: N2O CO2 PCl5 SiO2 NI3 SeO B2O3 XeF4 SO3 O2F2 Section 4.1 Naming Binary Compounds C. Naming Binary Compounds: A Review Section 4.1 Naming Binary Compounds Binary Compounds What are the names of these compounds? (Use your machine to identify the type first) CuO Ag2S SrO ClF B2O3 Na3P TiCl4 MnF2 K2S NH3 OF2 CuCl2