Group 7:redox, uses and halide tests.
advertisement

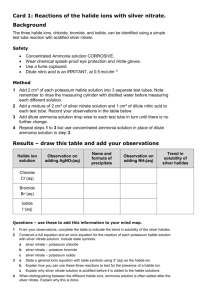
Periodicity is a regular periodic variation of properties of elements with atomic number and positions in the periodic table. Group 7:redox, uses and halide tests. • • • • L.O.: Explain the trend in boiling points of Cl2, Br2 and I2. Explain the trend in reactivity for the Group 7 elements. Describe the redox reactions of the Group 7 elements with other halide ions. Describe and interpret, using oxidation numbers, the reaction of chlorine with water and aqueous sodium hydroxide Electronic configurations of halogen atoms GROUP TRENDS OXIDISING POWER Cl2 + 2e- → 2Clhalogens are oxidising agents • they need one electron to complete their octet GROUP TRENDS OXIDISING POWER • halogens are oxidising agents • they need one electron to complete their octet • the oxidising power gets weaker down the group •Going down a group.. • increasing nuclear charge which should attract electrons more but this is offset by • INCREASED SHIELDING • INCREASING ATOMIC RADIUS The oxidising ability of the halogens decreases as we go up the group HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE Cl2(aq) + 2NaBr(aq) ——> Br2(aq) + 2NaCl(aq) HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE Cl2(aq) + 2NaBr(aq) ——> Br2(aq) + 2NaCl(aq) Cl2(aq) + 2Br¯(aq) ——> Br2(aq) + 2Cl¯(aq) HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE CHLORINE + SODIUM IODIDE BROMINE + SODIUM CHLORIDE IODINE + SODIUM CHLORIDE HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE CHLORINE + SODIUM IODIDE BROMINE + SODIUM IODIDE BROMINE + SODIUM CHLORIDE IODINE + SODIUM CHLORIDE IODINE + SODIUM BROMIDE HALOGENS - DISPLACEMENT REACTIONS THE DECREASE IN REACTIVITY DOWN THE GROUP IS DEMONSTRATED USING DISPLACEMENT REACTIONS... A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDE HALIDES ARE SALTS FORMED FROM HALOGENS (see next slide for examples) A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE e.g. CHLORINE + SODIUM BROMIDE CHLORINE + SODIUM IODIDE BROMINE + SODIUM IODIDE BUT BROMINE + SODIUM CHLORIDE BROMINE + SODIUM CHLORIDE IODINE + SODIUM CHLORIDE IODINE + SODIUM BROMIDE CHLORINE + SODIUM BROMIDE (Bromine is below chlorine in the Group so is less reactive) GROUP TRENDS OXIDISING POWER Chlorine oxidises bromide ions to bromine Cl2 + 2Br¯ ——> Br2 + 2Cl¯ Chlorine oxidises iodide ions to iodine Cl2 + 2I¯ ——> I2 + 2Cl¯ Bromine oxidises iodide ions to iodine Br2 + 2I¯ ——> I2 + 2Br¯ Look at Figure 4 in page 93 Disproportionation: a reaction in which the same element is both reduced and oxidised. Cl2(g) + H2O(l) → HClO(aq) + HCl(aq) Production of bleach: Cl2(aq) + NaOH(aq)→ NaCl(aq) + NaClO(aq) + H2O(l) L.O.: Describe the precipitation reactions of aqueous anions Cl–, Br– and I– with aqueous silver ions, followed by aqueous ammonia. Recognise the use of these precipitation reactions as a test for different halide ions. TESTING FOR HALIDES – AgNO3 • make a solution of the halide • acidify with dilute nitric acid – this prevents the precipitation of other salts. It get rids of carbonates and hydroxides. • add a few drops of silver nitrate solution • treat any precipitate with dilute ammonia solution • if a precipitate still exists, add concentrated ammonia solution TESTING FOR HALIDES – AgNO3 CHLORIDE white ppt of AgCl soluble in dilute ammonia BROMIDE cream ppt of AgBr insoluble in dilute ammonia but soluble in conc. IODIDE yellow ppt of AgI insoluble in dilute and conc. ammonia solution TESTING FOR HALIDES – AgNO3 CHLORIDE white ppt of AgCl soluble in dilute ammonia BROMIDE cream ppt of AgBr insoluble in dilute ammonia but soluble in conc. IODIDE yellow ppt of AgIinsoluble in dilute and conc. ammonia solution halides precipitate as follows Ag+(aq) + X¯(aq) ——> Ag+X¯(s) when they dissolve in ammonia a colourless diammine complex is formed [Ag(NH3)2]+(aq) TESTING FOR HALIDES – AgNO3 CHLORIDE BROMIDE IODIDE PLACE A SOLUTION OF THE HALIDE IN A TEST TUBE TESTING FOR HALIDES – AgNO3 CHLORIDE BROMIDE ADD SOME DILUTE NITRIC ACID IODIDE TESTING FOR HALIDES – AgNO3 CHLORIDE BROMIDE IODIDE ADD SILVER NITRATE SOLUTION WHITE PRECIPITATE OF SILVER CHLORIDE CREAM PRECIPITATE OF SILVER BROMIDE YELLOW PRECIPITATE OF SILVER IODIDE AgCl AgBr AgI TESTING FOR HALIDES – AgNO3 CHLORIDE BROMIDE IODIDE ADD DILUTE AMMONIA SOLUTION WHITE PRECIPITATE OF SILVER CHLORIDE CREAM PRECIPITATE OF SILVER BROMIDE YELLOW PRECIPITATE OF SILVER IODIDE - SOLUBLE - INSOLUBLE - INSOLUBLE TESTING FOR HALIDES – AgNO3 CHLORIDE BROMIDE IODIDE ADD CONCENTRATED AMMONIA SOLUTION WHITE PRECIPITATE OF SILVER CHLORIDE CREAM PRECIPITATE OF SILVER BROMIDE YELLOW PRECIPITATE OF SILVER IODIDE - SOLUBLE - SOLUBLE - INSOLUBLE




![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)