Name of compound
advertisement

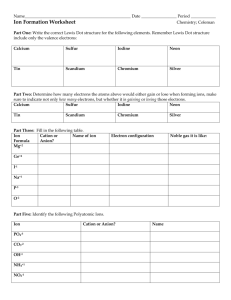
Unit 2: Antacids: adapted from Kendall Hunt Chemistry You Need To Know INTRODUCTION: Why do we need Antacids? Antacids Acid Inhibitors What do they do? How fast do they work Examples Section 2.1 Types of Matter What is matter? Can be separated Pure Substance Define/describe: Mixture Define/describe: Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Can be separated by chemical means Element Describe: Compound Can be separated by physical means Homogeneous Describe: Mixtures can be any combination of solids, liquids and gases: Combination Solid-Solid Solid-Gas: Solid-Liquid Liquid-Liquid: Gas-Liquid: Gas-Gas: True Solutions (homogeneous mixtures) o “Small” Particles do not settle out o Particles are too small to scatter light o Examples: Colloids: (heterogeneous mixture) o “Medium Sized Particles” do not settle out o Particles are large enough to scatter light (Tyndall Effect) o Examples: Standard & Honors Chemistry Example Heterogeneous Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Suspension: (heterogeneous mixture) o “Large” Particles will settle out over time. o Examples: Making Connections to Antacids o Consider an antacid like Maalox®, Tums, ®or Mylanta®. Are they pure substances or mixtures? o Antacids contain active ingredients and inactive ingredients. What purposes do inactive ingredients serve? List 4. You Try: Determine if the substance is an element, compound, homogeneous mixture or heterogeneous mixture. Aluminum foil (Al) Water( H2O) a glass of soda soil Carbon dioxide(CO2) Tap Water 2.2: Naming Chemicals The language of Chemistry includes element symbols, chemical formulas and chemical equations. Each element symbol starts with a capital letter. Ions An atom or group of atoms with a _______________________. The number of ________________________ does not equal the number of _____________________. WHY? Atoms ______________ or _______________ electrons to acquire stability like a noble gas. Monatomic Ions (formed from a single atom) Cations ________________ charge _______________ electrons Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Group A (Representative metals) :The group # for metals in columns 1A, 2A, and 3A is equal to the charge Naming them: Take the element name and add the word ion GroupB (Transition Metals): have more than one charge You should be familiar with the following transition metals with more than one charge. Fe+2 Fe+3 Sn+2 Sn+4 Pb+2 Pb+4 Co+2 Co+3 Cr+2 Cr+3 Mn+2 Mn+3 Cu+1 Cu+2 Naming them: Take the element name and place in parentheses the charge number as a roman numeral and add the word ion Anions ________________ charge ________________ electrons Group A (Representative metals) : subtract the group # for nonmetals in columns 4A, 5A, 6A, and 7A from 8 to get the charge. Naming them: Drop the ending of the element and add –ide and then ion Try These: Write the formula for each ion and name it. Calcium Aluminum Chromium Zinc Chlorine Iron Sulfur Phosphorus Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Binary Ionic Compounds Ionic bond: Binary Ionic Compound Ionic Compound METAL NONMETAL BINARY IONIC Identifying & Naming ionic compounds: Look for a compound with 2 elements, one metal and one nonmetal o o Write the name of the metals (cation) first. For the name of the anion, the ending of the element name is changed to “ide”. o Example: oxygen becomes oxide; sulfur becomes sulfide; fluorine become fluoride Examples: NaCl Cation: _______________ Name of compound: Anion: ________________ CaBr2 Cation: _______________ Name of compound: Anion: ________________ K2O Cation: ________________ Name of compound: Anion: _________________ Now you try: Complete the chart with the missing information. Formula Binary Ionic Compound name CaF2 Na3P NaCl SrBr2 Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Polyatomic Ions: more than one atom that together have a charge. Ternary Ionic Compounds: compound containing at least one polyatomic ion. polyatomic ionic compound Cation Polyatomic ion METAL POLTATOMIC ION TERNARY IONIC COMPOUND (Your reference table has a list of common polyatomic ions: you don’t need to memorize them.) Identifying polyatomic ions o The only cation (front-half) polyatomic ion is ammonium, NH4 o All other polyatomic ions are anions (written second in the formula) o Several polyatomic ions have the same three elements but the number of oxygen atoms is different between them so the subscripts on the oxygen atom will be different. Be careful to choose the correct one. o Example: sulfate SO4 and sulfite SO3 o If the formula contains parenthesis () the polyatomic ion is always inside the parenthesis. You Try: Find the polyatomic ion in the following compounds using your polyatomic ions list: NaNO3 NH4Cl Ca(OH)2 (NH4)3PO4 K2CO3 Naming compounds with polyatomic ions Look for a compound with more than 2 capital elements, at least one metal and one nonmetal o There will be more than 2 capital letters (none starting with H) o Write the name of the cation (the metal element or ammonium if cation is NH4) o If the polyatomic ion is the anion, then name it just as it is on the list. You do not have to change the ending of a polyatomic ion to “ide”. o If the anion is a single element and not a polyatomic ion, then the ending will change to “ide” . o Sometimes a compound is composed of 2 polyatomic ions. For these, you name the first polyatomic ion followed by the name of the second. Examples: Ca(NO3)2 Cation_ ______________ Name of compound: Anion: ________________ Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Na3PO4 Cation _______________ Name of compound: Anion: ________________ K2CO3 Cation: ________________ Name of compound: Anion: _________________ You Try! Formula Ternary Ionic Compound name Ca(C3H3O2)2 Li2SO3 Ba(OH)2 (NH4)2S Transition Metals (multi-valent metal). o Metal elements that have more than one possible charge for the cation. o a group “B” metal o Some of the most common transition metals are copper, iron, and nickel. o Although group B metals, SILVER (+1), CADMIUM (+2), and ZINC (+2) only have 1 charge o Although the elements of TIN and LEAD are group A elements, they do have more than one charge(Pseudo-transition) Sn(+2 and +4) and Pb(+2 and +4) Naming an Ionic Compound with a Transition Metal Look for a compound with one of the transition metals, excluding Ag, Cd, and Zn o Write the name of the metal element (cation) first. Write the name of the anion. Don’t forget the ending “ide” if it’s a single element. Leave a small space between for a Roman numeral. o To determine the Roman numeral to use, determine the total negative charge. Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know o Total negative charge = total positive charge for neutral compounds. o Divide total positive charge by the number of metal atoms o Write the charge in Roman numerals in parenthesis after the metal name. Examples: CuCl Fe2(CO3)3 ZnBr2 cation: cation: cation: anion: anion: anion: total negative charge: total negative charge: total negative charge: Roman numeral: Roman numeral: Roman numeral: Name: Name: Name: You try: Name the following compounds with transition metals. PbCl2 PbCl4 MnO Mn2O3 Covalent Compounds Covalent bond: Non metal Binary covalent compound: Non metal Covalent compound Identifying and Naming Covalent Compounds These compounds have 2 elements & both elements are non-metals Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know To Name a Binary Covalent Compound o o Write the name of the first non-metal with a prefix indicating the number of atoms. DO NOT USE the prefix “mono”. Write the name of the 2nd nonmetals with prefix indicating the number of atoms (Including the “mono” prefix if there is only one atom) and the suffix “ide” as the ending. Examples Prefix Monoditritetra pentahexaheptaoctanonadeca- Meaning 1 2 3 4 5 6 7 8 9 10 P2O5 SiF4 Prefix: Prefix: 1st element: 1st element: Prefix: Prefix: 2nd element: 2nd element: Name: Name: You try: NO2 N2O4 P4O10 CO Mixed Practice: Need to ask whether the compound is IONIC or COVALENT! Na2O Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know K3PO4 Cu(OH)2 N2S MgCl2 2.3 Writing Formulas for Chemical Compound Identifying Binary Ionic Compounds They end in “-ide” (except “hydroxide and cyanide”) & DO NOT contain covalent prefixes Writing formulas for Binary Ionic Compounds (metal element + non-metal element) o o o o o Write the symbol and charge for the cation (metal). Write the symbol and charge for the anion (non-metal). The sum of all of the positive charge and all of the negative charge equals zero in a neutral atom. If the total charge is not equal to zero, you must adjust the number of cations or anion to create a neutral compound. Use subscripts to show how many of each type of ion is there Note: Use your periodic table to help you determine the charges of most of the common cations and anions. Examples: Sodium chloride Sodium symbol & charge: Chlorine symbol & charge: Sum of the positive & negative charges: Formula: If we combined one of each ion we would write: NaCl. Adding up the charges would give an overall charge of zero since algebraically 1+-1 = 0. Since only one of each ion is needed we do not need any subscripts. Calcium bromide Calcium symbol and charge: Bromine symbol and charge: Sum of the positive & negative charges: Formula: Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know If we combined one of each ion we would write: CaBr . Adding up the charges would give an overall charge of +1 since algebraically 2+-1 = +1. An additional bromide ion is needed to make the charges equal to zero so a subscript of 2 is placed under the bromide ion. The Criss Cross Method is a simple way to determine the subscripts Write the symbol and charge for the cation (metal). Write the symbol and charge for the anion (non-metal). Cross the charge numbers down diagonally as subscripts if they are not equal to each other Simplify subscripts if you can Ca +2 Br-1 You try: Compound name Cesium chloride Cation symbol & Charge Anion Symbol & charge Formula Potassium oxide Calcium sulfide Lithium nitride Writing formulas for Polyatomic Ionic Compounds They DO NOT end in “-ide” (except “hydroxide and cyanide”) & DO NOT contain covalent prefixes The same process is used as for binary ionic compounds except the anion will be a polyatomic ion that has its own charge. (The exceptions are ammonium NH4 which will be cation.) o o o o o o Write the symbol for the cation (or the polyatomic ion symbol if ammonium or dimercury). Determine the charge of the cation. Write the symbol of the anion or polyatomic ion. Determine the charge. Adjust the number of each ion so that a neutral compound is formed by adding subscripts to indicate how many of each ion are needed if more than one. If you need more than 1 of the polyatomic ions, you must place parentheses around the formula for the polyatomic ion BEFORE you subscript it. The subscript goes outside the parenthesis. YOU DO NOT WANT 2 SUBSCRIPTS SIDE BY SIDE---MUST BE SEPARATED BY THE PARENTHESIS. Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Examples: Sodium Carbonate Sodium symbol & charge: Carbonate symbol & charge: Sum of the positive & negative charges: Formula: Magnesium nitrate Magnesium symbol & charge: Nitrate symbol & charge: Sum of the positive & negative charges: Formula: You try: Sodium nitrate Calcium chlorate Potassium sulfite Calcium hydroxide Writing formulas for compounds with multi-valent metals (transition metals) These compounds will have roman numerals. The same rules apply as for the compounds above. The Roman numeral gives you the charge on the cation or multi-valent metal (transition metal). Examples: Iron (III) oxide Iron symbol & charge: Oxygen symbol & charge: Sum of the positive & negative charges: Formula: Copper (II) nitrate Copper symbol & charge: Nitrate symbol & charge: Sum of the positive & negative charges: Formula: Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know You try: Iron (II) nitrate Copper (I) chloride Lead (IV) hydroxide Tin (II) oxide Writing formulas for BINARY COVALENT COMPOUNDS These compounds contain prefixes in their names. To write these formulas: The prefixes indicate how many of each atom that you need. If there is not a prefix is it understood to be 1. YOU DO NOT HAVE TO WORRY ABOUT CHARGES FOR THESE. NO CRISS CROSS METHOD EITHER! Examples: dinitrogen tetraoxide Silicon dioxide You try: Carbon monoxide Nitrogen dioxide Dinitrogen pentoxide NOTE: In chemistry “di” and “bi” prefixes do not refer to the same thing. Use “di” in naming compounds to indicate that 2 atoms are needed in a covalent compound. When “bi” is used in a name it means that the polyatomic ion contains a hydrogen atom. Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Mixed Practice: Write the formulas for the following chemicals Magnesium hydroxide Copper (II) nitrate Iron (III) oxide Nitrogen dioxide Sodium bicarbonate 2.4 Defining, Naming and Writing Acids and Bases Arrhenius Acids o Produce ________________________________________in water o The hydronium ion is a hydrogen ion attached to ________________ o To recognize and ACID, look for a hydrogen ion as the first element in an aqueous covalent compound. Naming non-oxygen acids (Binary Acids) These compounds start with “____________ & DO NOT contain______________. To name these compounds: o Use HYDRO _____________IC acid o Fill in the blank with the __________________name of the anion Example: HBr Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Naming Oxygen Acids (oxyacids) These compounds start with “_______” & contain ________________ To name these compounds: o Use __________ IC acids for “________________” anions o Use ________ OUS acids”for “_________________” anions Examples: H2SO3 H2SO4 You try: HCl HNO3 H2S H3PO4 Writing formulas for Acids o Acids with “hydro” in the name To write these formulas Write the cation ______ . Write the symbol for the anion and find its charge. Balance the charges by adding the appropriate ________________to the hydrogen cation if needed. Example: Hydrofluoric acid o Acids without “hydro” in the name To write these formulas Write the cation H+1 If it is an “IC ” acid, the anion is the “-ate ” polyatomic ion. If it is an “OUS ” acid, the anion is the” -ite” polyatomic ion. Add _________________to the hydrogen cation to _________________ charges. Example: Carbonic acid Nitrous acid You try: Phosphoric acid Hydroiodic acid Carbonous acid Perchloric acid Arrhenius Bases o Produces _________________________in water Naming Bases o Most bases are just __________________________with “hydroxide” as their anion. o The most common exception to this is _______________________. It is a base even though it does not contain OH- as the ________________. Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Example: NaOH You try: Ca(OH)2 KOH Sr(OH)2 Copper(II)hydroxide Magnesium hydroxide 2.5 Characteristics of Acids and Bases Acids Produces: Produces: Tastes: Tastes: Reacts with : Feels: Bases Both form ions when dissolved in water. They are called _____________________ Strength VS Concentration Strong: Weak: Dilute: Concentrated: Standard & Honors Chemistry Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know Combinations of Strength and Concentration Dilute Concentrated Strong Weak pH scale o o A scale that measures the acidity of a sample Ranges from : 1----------------------------------7-----------------------------------14 Acidic Neutral Basic o Most acidic: _______ o Most basic: _______ o Neutral: __________ Measuring pH Indicators change color based on pH o Liquid indicators examples are phenolphthalein or bromothymol blue) phenolphthalein Acid : clear Base: pink bromothymol blue Acid: yellow Base: blue o Paper indicator examples are litmus or pH paper o pH paper allows a color to match to a specific pH number For LITMUS paper: BLUE RED o pH probes or pH meters : Common substances and their pH values stomach acid vinegar Standard & Honors Chemistry grapes milk Unit 2: Antacids: Guided Notes: adapted from Kendall Hunt Chemistry You Need To Know saliva Standard & Honors Chemistry blood ammonia