Neuro Drugs Combined Inc Pain
advertisement

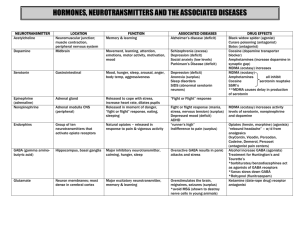
Anti-epilepsy Agents Dr Andrew Mallon Aims To describe the pathophysiology of epilepsy To determine the pharmacological agents used Mechanism of action Contra-indications Adverse effects Patient management Introduction 1 person in 20 will have an epileptic seizure at some time in their life Epilepsy is diagnosed on the basis of two or more epileptic seizures. Around 450,000 people in the UK have epilepsy (40 million people worldwide) A seizure is triggered by a sudden interruption in the brain's highly complex electro-chemical activity (National Society for Epilepsy UK) Age/Incidence Brain 100 billion neurons Control centre: Taken from OUP Illustration Resource, 2002 temperature sensory input motor control emotion thought? body functions Gross anatomy Front part of the brain; involved in planning, organizing, problem solving, selective attention, personality and a variety of "higher cognitive functions" including behavior and emotions. Gross anatomy The parietal lobes contain the primary sensory cortex which controls sensation (touch, pressure). Gross anatomy Region in the back of the brain which processes visual information. Gross anatomy These lobes allow a person to distinguish smells and are believed to be responsible for short-term memory. Structure Action Potential Synapse Activity Seizures are a symptom of an underlying CNS dysfunction It is an abnormal, uncontrolled electrical discharge from neurons Cell membrane disruptions (permeability) Altered ion distributions (chemical balance) Decreased neurotransmitters (Ach and GABA) Everyone has seizure threshold Classification of Seizures Partial: Simple partial seizures (no loss of consciousness) With motor symptoms With sensory symptoms With autonomic symptoms Only involve 1 hemisphere Dependant on which area of the brain Complex partial (loss of consciousness) Simple followed by loss of consciousness Impaired at the onset Unclassified: Classification not possible to problems with diagnosis – suspected Generalised (affect whole brain with loss of consciousness): Clonic, tonic (1min) or tonic-clonic (2-4min): muscle spasm (extensors), respiration stops, defecation, salivation, violent jerks Myoclonic: seizures of a muscle or group of muscles Absence: Abrupt loss of awareness of surroundings, little motor disturbance, mostly children Atonic: loss of muscle tone/strength Pathological Basis Abnormal electrical discharge in the brain Coordinated activity among neurons depends on a controlled balance between excitation and inhibition Any local imbalance will lead to a seizure Imbalances occur between glutamate-mediated excitatory neurotransmission and gammaaminobutyric acid (GABA) mediated inhibitory neurotransmission Generalised epilepsy is characterised by disruption of large scale neuro-networks in the higher centres. Normal Processes Depolarising Na+ and Ca++ ionic current shifts are activated by glutamate receptors Repolarising K+ currents are mediated by GABA receptors Hyperpolarisation is mediated by GABAa receptors creating an influx of Cl- => inhibition of impulse generation. Any defect causes the neuron to be closer to the all or none threshold for an AP = HYPEREXCITABLE STATE. Leading to instability between excitation and inhibition => Epilepsy Other possible causes Inherited mutations of proteins involved in the ion channels Reduction in the activity of homeostatic ATPase pumps within neuron cell membranes Basis of Pharmacological Rx Most anti-epileptic agents act either by blockade of depolarisation channels (Na+ and Ca++) OR Enhancing the activity of GABA (neurotransmission inhibition) 5 Categories of Anti-epileptic Drugs All classifications are based upon chemistry: Hydantoins Succinimides Benzodiazepines Barbiturates Miscellaneous Hydantoins - Phenytoin (Dilantin) Use for pts with Tonic-Clonic seizures Acts to promote intracellular removal of sodium during the refractory period Antagonism (blocking) of Na+ channels to reduce excitability Antagonism of Ca++ channels Potentiation (activation) of GABA receptors to promote the inhibitory role of GABA Can be used in the Rx for neuropathic pain and cardiac arrhythmias Pharmacokinetics: Slowly absorbed from gut, use a slow IV if rapid action is required Avoid IM – muscle damage Eliminated by hepatic biotransformation Can measure amount of free agent in the saliva Cautions: hepatic impairment, pregnancy, breast-feeding; avoid sudden withdrawal; Blood or skin disorders Adverse effects: nausea, vomiting, mental confusion, dizziness, headache, tremor, transient nervousness, insomnia occur commonly; rarely dyskinesias, peripheral neuropathy; ataxia, slurred speech, nystagmus and blurred vision are signs of overdosage; rashes (discontinue; if mild re-introduce cautiously but discontinue immediately if recurrence), gingival hypertrophy and tenderness, coarse facies, acne and hirsutism, fever and hepatitis; lupus erythematosus, Stevens-Johnson syndrome, toxic epidermal necrolysis, polyarteritis nodosa; lymphadenopathy; rarely haematological effects, including megaloblastic anaemia (may be treated with folic acid), leucopenia, thrombocytopenia, agranulocytosis, and aplastic anaemia; plasma-calcium concentration may be lowered (rickets and osteomalacia) Dose: By mouth, initially 3–4 mg/kg daily or 150–300 mg daily (as a single dose or in 2 divided doses) increased gradually as necessary (with plasma-phenytoin concentration monitoring); usual dose 200–500 mg daily (exceptionally, higher doses may be used); child initially 5 mg/kg daily in 2 divided doses, usual dose range 4–8 mg/kg daily (max. 300 mg) Contraindications: increases metabolism of the contraceptive pill, anti-coagulants, and pethidine Succinimides – Ethosuximide (Zarontin) Use for pts with Absence seizures Acts by antagonising Ca++ channels in the thalamocortical relay neurons => prevention of synchronised neuronal firing => raising AP threshold Pharmacokinetics: Almost complete absorption from the gut Extensive metabolism in the liver with a long half-life (2-3 days) Plasma and salivary concentrations correlate well for monitoring purposes Cautions: hepatic and renal impairment; pregnancy and breast-feeding; Adverse effects: gastro-intestinal disturbances, weight loss, drowsiness, dizziness, ataxia, dyskinesia, hiccup, photophobia, headache, depression, and mild euphoria. Psychotic states, rashes, hepatic and renal changes (see Cautions), and haematological disorders such as agranulocytosis and aplastic anaemia occur rarely (blood counts required if signs or symptoms of infection); systemic lupus erythematosus and erythema multiforme (Stevens-Johnson syndrome) reported; other side-effects reported include gum hypertrophy, swelling of tongue, irritability, hyperactivity, sleep disturbances, night terrors, inability to concentrate, aggressiveness, increased libido, myopia, vaginal bleeding Dose: avoid sudden withdrawal Blood disorders (review) adult and child over 6 years initially, 500 mg daily, increased by 250 mg at intervals of 4–7 days to usual dose of 1–1.5 g daily; occasionally up to 2 g daily may be needed; child up to 6 years initially 250 mg daily, increased gradually to usual dose of 20 mg/kg daily Contraindications: may make tonic-clonic seizures worse Bensodiazepines – Clorazepam (Klonopin), Diazepam (Valium) Act by potentiating the actions of GABA causing neurotransmission inhibition (primarily in the CNS) Can be used to induce sleep (high dose), anticonvulsant therapy and reduction in muscle tone. Pharmacokinetics: Well absorbed from the gut Lipid soluble to ensure ready prentration of the blood brain barrier Metabolised in the liver to create active agents (prolonged therapeutic action) Slow elimination from body Eg Clonazepam Cautions: elderly and debilitated, respiratory disease, spinal or cerebellar ataxia; history of alcohol or drug abuse, depression or suicidal ideation; myasthenia gravis; porphyria; hepatic impairment; renal impairment; pregnancy; breast-feeding Contra-indications: respiratory depression; acute pulmonary insufficiency; sleep apnoea syndrome; marked neuromuscular respiratory weakness including unstable myasthenia gravis Adverse effects: drowsiness, fatigue, dizziness, muscle hypotonia, co-ordination disturbances; also poor concentration, restlessness, confusion, amnesia, dependence, and withdrawal; salivary or bronchial hypersecretion in infants and small children; rarely gastrointestinal symptoms, respiratory depression, headache, paradoxical effects including aggression and anxiety, sexual dysfunction, urinary incontinence, urticaria, pruritus, reversible hair loss, skin pigmentation changes; dysarthria, and visual disturbances on longterm treatment; blood disorders reported; overdosage: Dose: 1 mg (elderly 500 micrograms) initially at night for 4 nights, increased according to response over 2–4 weeks to usual maintenance dose of 4–8 mg daily in 3–4 divided doses; may be given as a single daily dose in the evening once maintenance dose established; max. 20 mg daily; child up to 1 year, initially 250 micrograms increased as above to usual maintenance dose of 0.5–1 mg, 1–5 years, initially 250 micrograms increased as above to 1–3 mg, 5–12 years, initially 500 micrograms increased as above to 3–6 mg Barbiturates – Phenobarbital (Luminal) Used for tonic-clonic seziures. Act by increasing the duration of Cl- ion channel opening by activating neuronal GABAa receptors Causing hyperpolarisation of the AP, making it less likely to fire again Essentially, acts like GABA and can even potentiate the effects of GABA when present. Pharmacokinetics: Almost complete absorption Elimination is by heptic and renal (25% excreted unchanged) Biotransformed in the liver into 2 active metabolites Plasma concentrations relate poorly to seizure control, use only for monitoring of patient compliance. Adverse effects: CNS effects (sedation and fatigue) Restlessness/Hyperactivity Folate deficiency Tolerance Dependence with physical withdrawal reactions Adverse drug-drug reactions (contraception and warfarin). Contraindications: Do not use with patients with respiratory depression, children or elderly. NOTE: low therapeutic index means more toxic and overdose can have serious consequences Miscellaneous Agents – Carbamazepine (Tegretol) Used in most epilepsy types. MoA not fully understood but believed to be related to: Antagonist action of Na+ channels to inhibit repetitive neuronal firing Decreasing the production (or release) of glutamate (excitatory chemical) Can also be used in the Rx of neuropathic pain Pharmacokinetics: Slow and incomplete absorption Metabolised in the liver – creates an expoxide metabolite that can have a weak therapeutic effect Relatively long half-life (1-2 days) Potency decreases overtime therefore need to increase dose to ensure adequate control of seizures Plasma and salivary concentrations correlate well to clinical effectiveness Adverse effects: Nausea & vomiting (especially early Rx), constipation, diarrhoea and anorexia Skin irritation CNS toxcity – dizzy, drowsy, confusion Bone marrow depression (rare) Drug-drug reactions (contraception, warfarin) Contraindications: see drug-drug reactions. Sodium Valproate Use in all forms of epilepsy, as it suppresses the initial seizure discharge and its spread. Clinical actions are: Antagonism of Na+ and Ca++ channels Potentiation of GABA Attenuation of Glutamate Can be fast acting due to Na+ MoA, although the full Rx effect usually takes weeks. Pharmacokinetics: Well absorbed from gut (should be taken with food to counteract gastric irritation) Extensively metabolised in the liver Rapidly transported across the blood brain barrier Monitor plasma concentration for patient compliance only Adverse effects: GI upset (Nausea, vomiting, anorexia, abdominal pain and diarrhoea) Weight gain (appetite stimulation) Transient hair loss Tremor Coma (rare) Thrombocyptopenia (platelets) Oedema Severe hepatotoxicity (liver damage) Contraindications: People with liver damage or a history hepatic dysfunction Vigabatrin Only used in conjunction with other agents when pt becomes resistant (due to tolerance) or poorly tolerates Effective in partial epilepsy but with restricted used due to severe adverse effects (vision) MoA: completely different to other agents as it is a structural analogue of GABA that the enzyme that normally inactivates GABA will degrade instead of GABA. More GABA available to inhibit neuron transmission Pharmacokinetics: Rapidly absorbed from the gut Unchanged by renal processes Intermediate half-life (hrs) Blood concentrations are of no value. Adverse effects: Sedation, fatigue, dizziness, nervousness, irritability, depression, impaired concentration. tremor (CNS effects) Psychotic reactions (check pt history) Visual defects after prolonged use Weight gain and oedema Lamotrigine (Lamictal) Used for partial seizures in adults only Acts by the inhibition (antagonism) of neuronal Na+ channels but is highly selective (onlu neurons that synthesise glutamate and aspartate) Additionally, decrease glutamate release Pharmacokinetics: well absorbed, extensively metabolised in the liver and has a long halflife. Adverse effects: Fever, influenza-like symptoms Skin irritation GI disturbances (vomiting, diarrhoea) CNS effects (drowsiness, headache, dizziness, double vision) Contraindications: Pts with hepatic impairment Gabapentin (Neuronitin) Used for partial seizures in adults Designed to be a structural analogue of GABA but it does not mimic GABA in the brain. Acts via: Increased synthesis and release of GABA Decrease degradation of GABA Inhibition of Ca++ channels Pharmacokinetics: Adverse effects: Incompletely absorbed in the gut Excreted unchanged via kidney processes Short half-life CNS effects (dizzy, drowsy, fatigue, headache, double visions) Nausea and vomiting Contraindication: be careful with sudden withdrawal in the elderly due to kidney effects and alterations in acid-base balance. Anti-Parkinson Drugs Dr Andrew Mallon Aims To review pathogenesis of Parkinson's To review clinical presentation To identify treatment drugs Prevalence 1.5 million in USA and 120,000 in the UK – accounts for about 10% of all acute hospital admissions Effects 2 in 1,000 people; aged 80+ incidence is 1 in 50. Mainly affects adults in later life Slightly more common in men, Afro-Caribbean's and people from the Indian subcontinent Affects the quality of life of about 500,000 (family, carers etc) Causes Unclear, but is a number of factors: Environmental – toxins Free Radicals – there is a increase in post-mortem brain sections Aging – age related decline in dopamine production Genetic – possible, no single gene identified Parkinson’s Disease A degenerative and progressive disorder Associated with neurological consequences of decreased dopamine levels produced by the basal ganglia (substantia nigra) Dopamine is a neurotransmitter found in the neural synapses in the brain Normally, neurones from the SN supply dopamine to the corpus striatum (controls unconscious muscle control) Initiates movement, speech and self-expression Balance, posture, muscle tone and involuntary movement depends on the roles of dopamine (inhibitory) and acetylcholine (Ach: excitatory) If dopamine missing, Ach produces more of an effect on muscles Basis to exploit by drugs: Restore dopamine function Inhibit Ach within corpus striatum Consequences of dopamine reductions Tremors – hands and head develop involuntary movements when at rest; pin-rolling sign (finger and thumb) Muscle rigidity – arthritis-like stiffness, difficulty in bending or moving limbs; poker face Brandykinesia – problems chewing, swallowing or speaking; difficulty in initiating movements and controlling fine movements; walking becomes difficult (shuffle feet) Postural instability – humped over appearance, prone to falls Additional symptomology Anxiety Depression Sleep disturbance Dementia Disturbance of ANS (difficulty in urinating) Clinical Presentation Altered body image (depression) Poor balance Bradykinesia (slow movement) Bradyphrenia (slowness of thought) Constipation Dribbling/drooling Dyskinesias (involuntary movements) Dysphagia (difficulty swallowing Dystonia (pain spasms) Excessive sweating (impaired thermoregulation) Festinating gait Hullucinations (visual) Postural hypotension Restless leg syndrome (leg aches, tingle, or burn) Rigidity Sleep disturbance Slurring/slowing of speech Tremor Ref: Noble C (2000) Parkinson’s Disease – the challenge. Nursing Standard, 15 (12), 43-51 Videos GO TO MEMORY STICK Treatment (early stage) Clinical judgements based upon level of disability, age, cognitive status, concurrent medial problems Initial pharmacological therapies are titrated to determine optimal dose per person Agent used: Levodopa Social support and health education vital Referrals to other professional groups (SLT, PT, OT etc) Treatment (maintenance stage) Speech therapist is prophylactic and deals with swallowing problems (recommend exercises etc) Impaired thermoregulation – use beta-blockers Disturbance in sleep – can be side effects of medication; change time of intake or use a controlled release drug delivery system Continued health education and liaison with other professionals Treatment (complex stage) Function has deteriorates to such a level a combination of drugs are prescribed Dyskinesias and Dystonia – can be associated with long-term Levodopa use and it can be difficult to manage these effects – co-agent is co-beneldopa Restless-leg – dopamine agonists Anxiety – relaxation, distraction, CBT Depression – alterations in dose of anti-parkinson’s drugs Cognitive problems – referral to clinical psychologist and prescription of anti-dementia agents Hallucinations - ?anti-psychotics Essentially, a multidimensional approach to pharmacological treatment combined with a multidisciplinary approach Medication Rational Replace depleted levels of dopamine Stimulate the nerve receptors enabling neurotransmission Increase the effect of dopamine on nerve receptors (agonist) Counteract the imbalance of Ach and Dopamine The Drugs: Dopaminergic drugs (improving dopamine functioning) Levodopa Dopamine receptor agonists Amantadine Selective monoamine oxidase B inhibitors Catechol-O-methyltransferase inhibitors Antimuscarinic drugs (Ach inhibitors) Levodopa (or Levodopamine) Can not administer dopamine directly, as it does not cross the blood brain barrier A natural amino acid that the brain converts into dopamine (replacement therapy) used since the 1960’s To make it slow release, combined with benserazide (an enzyme inhibitor) to create co-beneldopa or cocareldopa (Sinemet) Dose = 50, 100 or 200mg (12.5, 25 or 50mg) Source: Adams et al (2006). Pharmacology for Nurses – A Pathophysiologic Approach. Prentice Hall Publishers Pharmacokinetics: Absorbed by the small intestine by an active transport system Decarboxylation occurs in peripheral tissues (gut wall, liver and kidney decrease amount available for distribution – 1% of an oral dose Extracerebral dopamine amounts causing unwanted effects (benserazide) Short half-life Cautions: pulmonary disease, peptic ulceration, cardiovascular disease, diabetes mellitus, osteomalacia, open-angle glaucoma, history of skin melanoma (risk of activation), psychiatric illness (avoid if severe); warn patients about excessive drowsiness; in prolonged therapy, psychiatric, hepatic, haematological, renal, and cardiovascular surveillance is advisable; warn patients to resume normal activities gradually; avoid abrupt withdrawal; Contra-indications: closed-angle glaucoma; pregnancy breast-feeding Adverse effects: anorexia, nausea and vomiting, insomnia, agitation, postural hypotension (rarely labile hypertension), dizziness, tachycardia, arrhythmias, reddish discoloration of urine and other body fluids, rarely hypersensitivity; abnormal involuntary movements and psychiatric symptoms which include hypomania and psychosis may be dose-limiting; depression, drowsiness, headache, flushing, sweating, gastro-intestinal bleeding, peripheral neuropathy, taste disturbance, pruritus, rash, and liver enzyme changes also reported; syndrome resembling neuroleptic malignant syndrome reported on withdrawal Dose: Initially 125–500 mg daily in divided doses after meals, increased according to response (but rarely used alone, see notes above) HOMEWORK: WHAT DRUGS INTERACT WITH LEVODOPA? Adverse effects: As a result of the amount of peripheral dopamine levels Nausea, vomiting Postural hypotension As a result of the amount of CNS dopamine levels Dyskinetic involuntary movements (face & neck) Hallucinations and confusion Dopamine receptor agonists Apopmorphine (APO-go): SC administration Rescue therapy – rapid onset with a short duration of action (~50mins) Bromocriptine (Parlodel); Pergolide (Celance); Ropinirole (Requip) Direct agonists of dopamine receptors in the brain ?longer lasting therapeutic effects that Levodopa Start a pt on this alone, then combine with levodopa to ‘smooth out’ control when PD is getting progressive (especially young) Pharmacokinetics: Incompletely abosrbed need extensive first-pass metabolism (biotransformed in liver) Pergolide & Ropinirole have higher bioavailability (distribution) Short to medium half life (Potency) Adverse effects: Use gradual dose titration N + V (particularly Apomorphine) Dyskinesia Hallucinations and confusion Peripheral vasospasm (Raynaunds) Respiratory depression (Apomorphine Amantadine (Symmetrel) Originally an antiviral drug, now used as conjucntive therapy for dyskinesis effects produced by Levodopa MoA: Pharmacokinetics: stimulates/promotes the release of dopamine stored in the synaptic terminals Reduces reuptake of released dopamine by pre-synaptic neuron Well absorbed, long half-life, excreted unchanged by the kidney Adverse effects: Not many Ankle oedema, postural hypotension, nervousness, insomnia, hallucinations (high dose) Other Disease Modifying Drugs Selective monoamine oxidase B inhibitors (selegiline – Trade name Eldepryl/Zelapar): MoA: prolongs the effects of levodopa as MAO-B degrades dopamine Pharmacokinetics: completely absorption, short half-life Adverse effects: N, V, Dia, Constipation; dry mouth, sore throat; transient dizziness; insomnia, confusion and hallucinations Early stage – prescribed on it is own to delay need for levodopa and there is good evidence for its slowing down of PD progression Catechol-O-methltransferase inhibitors - COMT (entacapone, Trade name Comtess) MoA: inhibits the breakdown of levodopa Pharmacokinetics: variability of absorption, extensive first-pass metabolism, short half-life Adverse effects: dyskinesias, hallucinations; N, V, Dia and abdominal pain New combination – Levodopa/carbidopa/entacapone (Stalevo) as 1 tablet (50, 100, 150mg) Antimuscarinic/Anticholinergic Drugs: Trihexyphenidyl (Broflex, Artane, Agitane); Benztropine (Cogentin); Orphanadrine (Disipal); Procycline (Kemadrin, Arpicolin) Less common drugs but they affect Ach based interactions MoA: blocking cholingeric (Ach) receptors to restore balance Pharmacokinetics: fairly well absorbed, extensive hepatic metabolism, intermediate to long half-lifes Adverse effects: dry mouth and confusion Disease Modifying Drugs Overview Symptom Management Drugs PD is multidimensional, therefore there are a number of clinical presentations that require supplementary agents Drug-Drug reactions is the problem Major area is depression Antidepressants Amitriptyline (Tryptizol), imipramine (Tofranil), Nortriptyline (Allegron), Iofepramine (Gamanil) MoA: block re-uptake of noradrenaline and serotonin => Sedative actions, can help with drooling and loss of appetite Adverse effects: sleepiness, dry mouth, increased hunger, cardiac arrhythmias and changes in BP Can interfere with the effects of levodopa! Other Drugs to Avoid Generic Name Brand Name Prescribed for Prochlorperazine Prephenazine Flupentixol Stemetil Triptafen Fluanxol/Depixol Chlorpromazine Largactil Pimozide Sulpiride Orap Dolmatil N +V, Dizziness Depression Confusion, Hallucinations “ “ “ Video Sites HealingWell.com Birmingham Teaching Tutorials (hopefully) The Neuron Connection www.bio.davidson.edu/projects/neuron/video.asp Useful Websites: Parkinson’s Disease Society http://www.parkinsons.org.uk/ Nursing Standard (CPD) http://www.nursing-standard.co.uk/ Pathophysiology of Pain Dr Andrew P Mallon Context “It is easier to find men who will volunteer to die, than to find those who are willing to endure pain” Julius Caesar “We all must die. But if I can save (a person) from days of torture, that is what I feel is my great and ever new privilege. Pain is a more terrible lord of mankind than even death itself” Albert Schweitzer, 1953 Aims To define pain and develop an operational definition. To explore the physiological processes involved (from cause to perception). To examine the role of opioids in pain modulation Can we define pain? ‘Noxious stimuli via nociceptors which are free nerve endings found in skin, muscle and joints which transmit impulses the brain’ (Bakal 1974) ‘Abnormal experience evoked by abnormal, harmful or noxious stimuli’ (Wyke 1981) ‘A subjective combination of sensory, emotional and cognitive factors’ (Bond 1984) ‘Sensory and emotional experience of discomfort, which is usually associated with threatened tissue damage’ (Sander 1985) ‘Pain is whatever the patient says it is’ (Sternbach & McGaffey 1974) What is Pain? Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. International Association for the Study of Pain (1991). What is Pain? Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. International Association for the Study of Pain (1991). Aspects….. Physiology Psychology Social Relates to: sensory cognitive affective (emotion) Pain Classification Acute and Chronic Underlying cause transitory or protracted/ongoing Fast and Slow Pain Sharp and dull/throbbing Pain = Sensation 1. Transduction of noxious stimuli by sensory receptors 2. Transmission of nociceptive information 3. Perception of noxious information 4. Modulation of the incoming noxious information Basic pathway Periphery Enters spinal cord Travels up towards the tertiary Interneuron Secondary Interneuron brain Medulla Thalamus (processor) Cerebral cortex Primary Interneuron Nociceptive pathways (Walsh, 1997) Somatosensory cortex and limbic system Perception Supraspinal structures: Thalamus Midrain/Pons/Medulla Basal Ganglia Spinal Cord: Pain pathways Dorsal horn synapses Peripheral Nerve Fibres: A-delta C-fibres Cortical Level Supraspinal Level Spinal Segmental Level Nociceptor: Detection Transduction Nociception Peripheral Level Noxious Stimulus Peripheral Level Noxious stimulus: Bradykinin Histamine Potassium/ Protons (H+) Prostaglandins Substance P Serotonin Nociceptor Activation: Transducer to convert the stimulus Receptor potential => Action potential Activity in ascending sensory pathways (Tortora & Grabowski, 2004) Peripheral Nerve Fibres Name Diameter NCV AP Sensory Duration Function A-Alpha 20 micron 70-120 ½ ms Proprioms ception A-Beta 10 micron 30-70 ½ ms Touch ms A-Delta 3 micron 12-30 1 ms Sharp ms Pain C-fibres 0.5 micron ½-2 ms 2 ms Slow Pain (Tortora & Grabowski, 2004) Afferents into laminas 1,2,5, plus motor Afferents into laminas 1,2,5, plus motor & autonomic Ascending Nociceptive Pathways Lateral Spinothalamic: arises in Lamina I & V peripheral input from A units respond to high threshold noxious stimuli & non-noxious stimuli synapses in thalamus collateral's to midbrain Multi-synaptic spinoreticular arises in lamina VII & VIII input from most other lamina Collateral's to brain stem reticular formation projects to thalamus Spinothalamic Tract (Almeida et al., 2004) Spinoreticular Tract (Almeida et al., 2004) Limbic System Nociceptors Hypothalamus Frontal Lobe Somatosensory Cortex/Insular Thalamus Reticular Formation C fibres Spinal Cord Medulla/Pons A delta fibres (Adapted from Johnson, 1997) Pain Modulation Ability by either external or internal influences or “interference” to alter the perception of a painful experience Non-pharmacological (physical therapy, acupuncture etc) Pharmacological “Mind over matter” Placebo? Therapist interaction effect Gate control (spinal level) LDA + - - T + SDA Gate Control System Action system Supraspinal Descending Pain Inhibitory Pathways The inhibitory interneurones in the substantia gelatinosa may also be influenced by descending inputs from higher centres. Gate control (spinal level) Reticular formation/ Thalamus LDA + - - - T + SDA Gate Control System Action system Descending Pain Pathways Descending inhibitory control LDA + T SDA Gate Control System Action system Endogenous Pain Modulating System Opioid Peptides enkephalins dynorphins endorphins Opioid Receptors widely distributed role in pain modulation role in neural tissue growth & in formation of new central synaptic connections variation in types of receptors variation in activity (agonist/antagonist) 3 major subtypes: μ (mu): PAG & SC κ (kappa): Peripheral δ (delta): Throughout Exogenous Opioids Work by binding to CNS endorphin receptors Activate descending pain pathways Specific site of action is at second order neuron level Each analgesic agent have different pattern of affinities => different effects/pain experiences Role of Opioids Exogenous Opioids Endorphin Binding to brain stem receptors AP to Dorsal Horn Enkephalin Release Inhibition Pain Medication Weak Opioids (MSk Pain): Codine: 10% converted into morphine by liver, therefore used with paracetamol Tramadol: stimulation of the descending nerves from the brain which inhibit the dorsal horn of the spinal cord Strong Opioids: Morphine: is the "gold standard". It is a potent stimulator of morphine receptors, blocking pain impulses at several sites: in inflamed peripheral tissues (e.g. knee osteoarthritis), in the dorsal horn of the spinal cord, centrally in the brain. Hydromorphone: 7.5 times more potent Cognitive Control Descending inhibitory control LDA + T SDA Gate Control System Action system Summary Pain is multidimensional Physiologically it is sequence of events from cause to perception There are 2 main pain fibres (a-delta and C) and 2 corresponding path pathways in the spinal cord (Spinothalamic and Spinoreticular) Pain modulation can occur endogenously and exogenously. Key concept: inhibition at the dorsal horn. Key References Almeida TF, Roizenblatt S, Tufik S (2004) Afferent pain pathways: a neuroanatomical review. Brain Research 1000: 40-56. Johnson MI (1997) The Physiology of the Sensory Dimensions of Clinical Pain. Physiotherapy 83(10): 526-536 Kidd BL (1996) Problems with pain - is the messenger to blame? Annals of the Rheumatic Diseases 55:275 Kidd BL, Morris VH, Urban L (1996) Pathophysiology of joint pain. Annals of the Rheumatic Diseases 55:276-283 Kidd BL (1999) What are the mechanisms of regional musculoskeletal pain? Bailliere’s Clinical Rheumatology 13(2):217-230 Wells P, Frampton V, Bowsher D (1994) Pain: management by physiotherapy. 2nd edn, Butterworth Heinemann, Oxford.