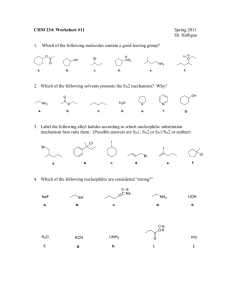
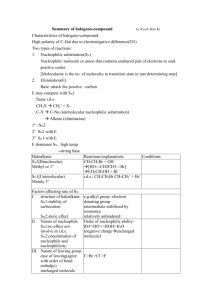
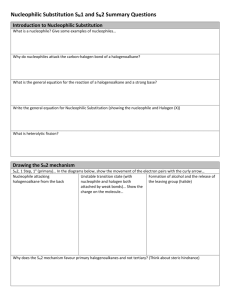
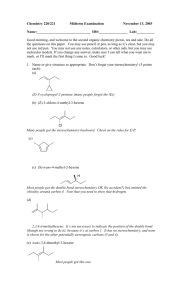
Substitution Reactions: Mechanisms
advertisement

Part 3 CHM1C3 Substitution Reactions R1 R1 Nu R1 Rate = k [R-Cl][Nu] Nu R3 2 R Inversion of Configuration Rate = k [R-Cl] Nu SN2 R3 2 R Cl R3 R2 R1 SN1 Nu 3 R R2 Racemisation of Configuration Content of Part 3 Part 3i. The Role of Kinetics and Chirality in Determining Mechanisms of the Substitution Reaction Part 3ii. Effect of Solvent on the Substitution Reaction Part 3iii. Effect of Structure on the Substitution Reaction Part 3iv. Nature of the Attacking Nucleophile Part 3v. Nature of the Leaving Group Part 5i Substitution Reactions: Mechanisms Bimolecular substitution (SN2) (and elimination (E2)) reactions and transition states Unimolecular substitution (SN1) (and elimination (E1)) reactions and reactive intermediates Content of Part 5i The SN2 Reaction Mechanism The SN1 Reaction Mechanism Reaction Rates/Chirality in Determining the Mechanism Transition States Reactive Intermediates CHM1C3 – Introduction to Chemical Reactivity of Organic Compounds– – Learning Objectives Part 5i – Substitution Reactions: Mechanisms After completing PART 4i of this course you should have an understanding of, and be able to demonstrate, the following terms, ideas and methods. (i) Understand how by considering both the reaction kinetics and the stereochemical outcome of substitution reactions the SN2 and SN1 mechanisms were devised, (ii) Understand the difference in timings of the arrow-pushing in the mechanisms of the SN2 and SN1 reaction, (iii) Understand the terms bimolecular and unimolecular, (iv) Understand the reaction energy profile for a reaction in which a transition state leads to the formation of the products – a SN2 reaction, and (vi) Understand the reaction energy profile for a reaction in which a reactive intermediate leads to the formation of the products – a SN1 reaction. Nucleophililic Substitution Reactions at sp3 Carbons R R Stereochemistry Rate X Nu R' Nu Equation R' "R X "R It is found that there are two possible stereochemical outcomes, each described by a different rate equation, and different stereochemical outcomes. Descriptor Rate Equation Stereochemical Outcome SN2 rate = k[R-Hal][Nu] Inversion SN1 rate = k[R-Hal] Racemisation Clearly, two different reaction mechanisms must be in operation. It is the job of the chemists to fit the experimental data to any proposed mechanism Reaction Mechanisms The mechanism of a reaction consists of everything that happens as the starting materials are converted into products. In principle, therefore, writing (or drawing) the mechanism means describing everything that happens in the course of the reaction. However, providing an exact description of a reaction on paper is an impossible goal. Instead, a proposal for the mechanism of a reaction should include certain types of information about the course of the reaction. Thus, the reaction mechanism should: [1] Account for the number of reaction steps as indicated by the rate equation [2] Account for reactive intermediates or transition states [3] Account for any stereochemical relationships between starting materials and products SN2 The SN2 Reaction Mechanism R Nu Bimolecular Process 1 Nucleophile attacks from behind the C-Cl s-bond. Cl R3 Rate = k[R-Hal][Nu] 2 R sp3 This is where the s*-antibonding orbital of the C-Cl bond is situated. Rate Determinig Step R1 1 – 2 Nu Cl R3 Bond Forming 1 – 2 R2 Bond Breaking Transition State – Energy Maxima sp2 R1 Nu R3 R2 Inversion of Configuration Cl http://chemistry.boisestate.edu/rbanks/organic/sn2.gif http://www.personal.psu.edu/faculty/t/h/the1/sn2.htm http://www.bluffton.edu/~bergerd/classes/CEM221/sn-e/SN2-1.html Transition States: See SN2 and E2 Reaction Mechanisms A transition state is the point of highest energy in a reaction or in each step of a reaction involving more than one step. The nature of the transition state will determine whether the reaction is a difficult one, requiring a high activation enthalpy (DG‡), or an easy one. Transition states are always energy maxima, I.e. at the top of the energy hill, and therefore, can never be isolated. A transition states structure is difficult to identify accurately. involves partial bond cleavage and partial bond formation. It Transition States Rate = k[A][B] Transition State Energy Maxima E n e r g y See SN2 and E2 Reaction Mechanisms [A.B]‡ DG‡ A+B DGo C+D Reaction Coordinate R1 1 – 2 Nu Cl R3 Bond Forming E n e r g y 1 – 2 R2 Bond Breaking Transition State – Energy Maxima R Nu R3 sp2 1 Cl 2 R R1 Nu R3 2 R Reaction Coordinate Cl SN1 The SN1 Reaction Mechanism Unimolecular Process R1 Cl R 3 R2 sp3 Rate = k[R-Hal] Rate Determining State Nucleophile attacks from either side of the carbocationic intermediate. R1 Nu R3 R1 R2 Cl Nu R3 R1 R2 Reactive Intermediate – Energy Minima sp2 Nu R3 R2 Racemisation of Configuration Reactive Intermediates: Mechanisms See SN1 and E1 Reaction Reactive intermediates are energy minima, i.e. at the bottom of the energy hill, and therefore, can be isolated. A reactive intermediate structure is much easier to identify and in certain cases these high energy species can be isolated and structurally characterised. Reactive Intermediates Rate = k[A] Reactive Intermediate See SN1 and E1 Reaction Mechanisms And Radical Chain Reaction C+B EE nn ee rr gg yy DG‡ Energy Minima DG‡ A+B DGo D+E Reaction Coordinate R1 R3 E n e r g y R1 Reactive Intermediate R1 R3 R2 Nu R3 2 Cl R 2 R R1 R3 Reaction Coordinate Nu R2 CHM1C3 – Introduction to Chemical Reactivity of Organic Compounds– – Summary Sheet Part 3i – Substitution Reactions: Mechanisms The difference in electronegativity between the carbon and chlorine atoms in the C-Cl sigma (s) bond result in a polarised bond, such that there is a partial positive charge (+) on the carbon atom and a slight negative charge (-) on the halogen atom. Thus, we can consider the carbon atom to be electron deficient, and therefore electrophilic in nature (i.e. electron liking). Thus, if we react haloalkanes with nucleophiles (chemical species which have polarisable lone pairs of electrons, which attack electrophilic species), the nucleophile will substitute the halogen atom. The difference in electronegativity between the carbon and chlorine atoms in the C-Cl sigma (s) bond result in a polarised bond, such that there is a partial positive charge (+) on the -carbon atom and a slight negative charge (-) on the halogen atom, which in turn is transmitted to the -carbon atom and the protons associated with it. Thus, the hydrogen atoms on the -carbon atom are slightly acidic. Thus, if we react haloalkanes with bases (chemical species which react with acids), the base will abstract the proton atom, leading to carbon-carbon double bond being formed with cleavage of the C-Cl bond. Substitution (and elimination) reactions can be described by two extreme types of mechanism. One mechanism is a concerted and relies on the starting materials interacting to form a transition state, and the other is a step-wise process in which one of the starting material s is converted into a reactive intermediate, which then reacts with the other reagent. Discussions of transition states and reactive intermediates in the course of a reaction is very useful when proposing an organic reaction mechanism, which takes into account the experimental evidence for a reaction, such as rate equations and stereochemical outcomes. Exercise 1: Substitution Reactions cis-1-Bromo, 3-methylcyclopentane reacts with NaSMe (MeS— is an excellent nucleophile) to afford a product with molecular formulae C7H14S. The rate of the reaction was found to be dependent on both the bromoalkane and the NaSMe. (i) (ii) Identify the product, and propose an arrow pushing mechanism to account for the product formation. Answer 1: Substitution Reactions cis-1-Bromo, 3-methylcyclopentane reacts with NaSMe (MeS— is an excellent nucleophile) to afford a product with molecular formulae C7H14S. The rate of the reaction was found to be dependent on both the bromoalkane and the NaSMe. (i) (ii) Identify the product(s), and propose an arrow pushing mechanism to account for the product formation. Starting material molecular formula = C6H11Br Me Product molecular formula = C7H14S Br Lost Br, Gained SMe, Substitution Reaction Rate equation indicates bimolecular process, SN2 Br Me Br Me Me SMe MeS MeS Envelope Conformation of Cyclopentane Me SMe Exercise 2: Substitution Reactions Compounds A and B when treated with a weak base are deprotonated to form the carboxylate anion. One of these carboxylate anions then reacts further to afford the lactone P, whilst the other carboxylate anion is does not lead to P. Identify the carboxylate anion which affords P, and rationalise its formation with an arrow pushing mechanism, as well as rationalising why the other carboxylate anion does not afford P. I HO A O I HO B O O P O Answer 2: Substitution Reactions Compounds A and B when treated with a weak base are deprotonated to form the carboxylate anion. One of these carboxylate anions then reacts further to afford the lactone P, whilst the other carboxylate anion is unaffected. Identify the carboxylate anion which affords P, and rationalise its formation with an arrow pushing mechanism, as well as rationalising why the other carboxylate anion does not afford P. I A HO B I O HO O O Base Base P Reaction must be SN2 type, because if O it was SN1 like the carbocation below would be generated from both S1 and S2. Therefore both S1 and S2 would afford P I O O I s* orbital of C-I bond O O H HO O