BIOLOGY 52/SECTION 6
advertisement

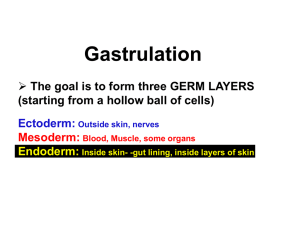
BIOLOGY 624 Fall 2009 DR. VICTORIA BAUTCH Lecture #5: Tues 9/22/09 DEVELOPMENTAL GENETICS GASTRULATION IV - Movements Reading: Gilbert, Ch8:215-216; Ch9:256-259; Ch10:295-302; Ch11:337-347. (Click here to access the Powerpoint) Paper discussion (Thurs 9/24): Sivak J, Petersen L, Amaya E. (2005). FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell 8, 689-701. A. GASTRULATION (literal meaning – to form a stomach) is a complex series of cell movements that: (Slide 2) 1. rearranges cells, giving them new neighbors (and thus potentially new signals from other cells and the environment) 2. results in the formation of 3 GERM LAYERS that will form the subsequent embryo: ECTODERM, ENDODERM, and MESODERM 3. forms the main body axes of the embryo (likely already specified, but the AP and D-V axes form at this time). 4. We’ll start with a summary of timelines of development in our different models to get started (Slide 4). The details of each timeline are in a supplemental section at the end of the lecture, along with the slides at the end of the Powerpoint. A few points a. These are lined up with gastrulation initiation, a framework suggested by O’Farrell et al. b. The overall developmental times are very different c. Vertebrate gastrulation initiation is well-defined, but the end is not well-defined. Gastrulation continues posteriorly while other processes such as neurulation and somitogenesis begin more anteriorly where gastrulation has been completed. B. Gastrulation can seem very different in different organisms (mostly due to geometric differences due to yolk content and distribution). 1 However, the similarities in terms of mechanisms and outcomes suggest that the same controls operate across species. Some movements that are combined in different ways during gastrulation are (Slide 5-11 (Slide 11 is CE movie): Note: we’ll skip some of this due to previous lecture coverage, but note that involution and ingression were mislabeled in previous lecture. We’ll go over a little more Convergent Extension since we discuss it later. 1. INVAGINATION – the infolding of a region of cells, like the indenting of a soft rubber ball when poked *2. INVOLUTION – the inturning or inward movement of an expanding outer layer so it spreads over the internal surface *3. INGRESSION – the migration of individual cells from the surface into the interior – the cells migrate independently 4. DELAMINATION – the splitting of one sheet of cells into two parallel sheets – the cellular movements are similar to ingression but the result is different 5. EPIBOLY – the movement of epithelial sheets that spread as a unit, rather than individually, to cover the deeper layers of the embryo. Cellular mechanisms of epiboly include cell division, cell spreading, and intercalation (several layers combining to form one layer). 6. CONVERGENT EXTENSION – the process whereby a group of cells changes from a “ball” to a “rod” shape – it is analogous to 4 lanes of traffic going down into one lane, but also results in lengthening of the long axis. The cellular mechanism is often intercalation. D. Gastrulation in the fly, Drosophila 1. It is useful to start a discussion of the movements of gastrulation by looking at a FATE MAP of the pre-gastrulation embryo. The fate map shows what the descendants of groups of cells will become. In this case, we see endoderm will form from each end, mesoderm from ventral cells, and ectoderm from lateral cells. The dorsal side is amnioserosa cells that will not be part of the final organism (Slide 12). 2. fly gastrulation begins at the mid-blastula transition, which signals the end of the cleavage cycles. The cells all have membranes and are distinct from one another. 3. First, about 1000 cells on the ventral side of the embryo invaginate to produce the VENTRAL FURROW (Slide 13). This furrow goes on to form a tube internally, which then flattens (Slide 14). 2 4. Next, the endoderm invaginates as two pockets at the anterior and posterior ends of the embryo (Slide 15). 5. Next, the ectoderm on the surface and the mesoderm undergo convergent extension, migrating toward the ventral midline to form the GERM BAND. The germ band includes all the cells that will form the trunk of the embryo. As this band extends it wraps over the back of the embryo and ends up next to the future head! (Slide 16 and 17) 6. During the GERM BAND EXTENDED phase, several key processes occur: a. organogenesis – for example, the nervous system forms by ingression of neuroblasts from the ectodermal sheet b. segmentation – each segment of the embryo, larva and adult fly has a unique identity relative to its placement on the A-P axis. We will learn later on how this occurs c. IMAGINAL DISC specification- these discs will survive pupation and make the adult fly. 7. The anterior and posterior endoderm invaginations form tubes that eventually meet and form the gut 8. The germ band retracts later so that the posterior segments are at the right end of the embryo. 9. Now let’s look at the whole process in living embryos with visual aids to orient us (SHOW MOVIE FROM FILE and VADE MECUM, Slide 18, 19) 10. Molecular Control of Fly Gastrulation a. Important signaling pathway is FGF. It was known that a FGF receptor called heartless (htl) was required for mesoderm movement, but the ligand was unknown. Not found in screens or when genome sequenced. Two groups in 2004 found 2 FGF genes closely linked that when deleted gave the same phenotype as htl mutants – mesoderm specified but not move (slide 20). --Further analysis showed that both genes regulated by Dorsal, and Levine group proposed a model for how receptor expressed in mesoderm while ligands expressed in neuroectoderm (slide 21). Also bring in transcription factors – twist and snail. E. Gastrulation in the frog, Xenopus 1. Again, let’s start by looking at a fate map to see what the progeny of the pre-gastrulation cells will become. The frog fate map shows that all the outside cells of the blastula (pre-gastrulation) will form either ectoderm or 3 endoderm, while the mesoderm will form from cells that are in the inner layer. (Slide 22). 2. Gastrulation starts by invagination of endoderm cells to form the BLASTOPORE LIP (Slide 23). This occurs 180o from the point of sperm entry, near the equator of the embryo. The dorsal blastopore lip has “organizer” activity (brief explanation here) – in this way this frog structure is analogous to Hensen’s node in the chick. The invaginating cells are called BOTTLE CELLS and have a distinctive shape (Slide 23). The cavity that forms is called the ARCHENTERON (the BLASTOCOEL cavity has already formed during the cleavage stage). 3. An important concept in gastrulation is that often there are sites that sequentially hold cells that originated at different places. Thus the cells that comprise the blastopore lip are constantly changing during gastrulation (Analogous structures in the chick and mouse are called the primitive streak). a. first the future endoderm cells go through the blastopore lip, and they will form the pharyngeal cells of the foregut later on (Slide 24) b. next, marginal zone cells involute that will form head mesoderm – these make the PRECHORDAL PLATE (Slide 24, 25) c. next, are mesoderm cells that will form the NOTOCHORD (a transient mesodermal “backbone” that is important for inducing and patterning the nervous system). (Slide 24, 25) d. subsequently the “lip” expands laterally, and heart, kidney and ventral mesoderm enter the embryo. 4. As cells enter the blastopore lip from the outside, the ectoderm spreads over areas vacated by the involuting cells. The ectoderm spread is EPIBOLY. This occurs by a combination of cell division and intercalation of several layers to make one layer. Eventually the ectoderm covers the whole yolk plug. (Slide 24, 25, 26) 5. As mesodermal cells enter the lip and migrate towards the future anterior pole or head, they undergo CONVERGENT EXTENSION, and become narrow in the future A-P axis while lengthening that axis (Slide 27). 6. Molecular Control of amphibian/zebrafish gastrulation – until very recently this aspect of gastrulation was a huge mystery. The complexity of the movements and the combination of movement with cell fate specification provided challenges. Some rather crude experiments implicated Fibronectin (FN) in some of the movements, but other effectors and the upstream initiators and regulators were unknown. This has been an area of intense study the last 5 years or so, and some quite interesting data has come from studies of frog and 4 zebrafish gastrulation to complement studies in the fly. The 3 areas that have seen breakthroughs are: --migration along the blastocoel roof --separation of migrating mesoderm from the ectoderm --the dramatic convergence extension (CE) movements of the dorsal surface – this third aspect has had the most exciting molecular evidence presented in the last few years so we will focus primarily on CE 1. Molecular Effectors – several molecules are thought to mediate the physical aspects of the movements – migration and convergent extension a. As described above, FN is important in providing the substrate upon which the involuting endoderm and mesoderm cells migrate (Slide 28). Davidson et al (2002) have shown that integrin alpha5 beta1 is an important FN receptor involved in this migration, and others have implicated alpha v integrin, another FN receptor. b. PAPC (par-axial protocadherin) is a cell surface adhesion molecule that appears to mediate convergent extension movements in both the frog (perhaps by interactions with Sprouty, see Wang et al, 2008) and in zebrafish, and a recent paper shows that PAPC is also required for tissue separation (Medina et al, 2004). --mutations prevent dorsal convergence of paraxial mesoderm (somites), and block tissue separation --overexpression promotes dorsal convergence, and can induce tissue separation c. Evidence for diffusible signals – if you condition tissue culture substratum with blastocoel roof explants, you promote directional migration, so what is involved? (Slide 29). 2. Molecular Initiators – what signals and pathways regulate the effectors? a. PDGF/PDGF receptor – this is important during frog gastrulation (Nagel et al, 2004). If the blastocoel roof explants have PDGFA knocked down, don’t support migration. Important isoform appears to be one that is linked to the matrix upon secretion. b. FGF/FGF receptor – important in avian gastrulation – migrating cells are attracted to FGF4 and repelled by FGF8, and expression patterns suggest they may be responsible for the lateral then medial movement of cells that ingress later in gastrulation (Slide 30, reviewed in Keller, 2005). 5 --Amaya’s group described a model that uses Sprouty and Spred proteins to toggle FGF signaling from cell fate information to morphogenetic information (Sivak et al, 2005) PAPER DISCUSSION FOR THURS. (Slide 30). c. Many studies show that a Wnt signaling pathway is critical for the execution of gastrulation. The canonical Wnt signaling pathway appears not to be involved, but rather a non-canonical pathway that is similar to the Planar Cell Polarity (PCP) pathway that polarizes cells in the horizontal axis in epithelial sheets (Huelsken and Birchmeier, 2001; Habas et al 2003) (Slide 31): --Convergence extension (CE) movements require Wnt signaling through Frizzled-7, and downstream activation of Rho and Rac (Slide 32). --However, the role of the Wnt signal may be permissive rather than instructive, since ectopic expression of Wnt allows for CE movements --CE also involves oriented cell divisions – that is to say, during gastrulation the dorsal ectoderm that undergoes CE has more divisions in the long axis than predicted from random orientation, and this process requires the PCP pathway (Gong et al 2004) (Slide 33) d. Transcription factors are upstream as well! --a T-box transcription factor called spadetail is upstream of PAPC in the zebrafish (Slide 34) --another transcription factor, Lim1, is also upstream of PAPC in Xenopus embryos (Hukriede et al, 2003). G. The end of gastrulation is not so dramatic as cleavage, and in fact in vertebrates it often continues at more posterior parts of the embryo, as neurulation commences at the anterior end. 1. After gastrulation, the basic body plan of the embryo is set up: head, tail, main body axis, back and belly. (Slide 35) 2. The 3 germ layers have formed and will go on to produce all of the embryonic structures except the germline: (Slide 36) --the ECTODERM is the most external layer and will produce skin, neural tube that becomes brain and spinal cord, and neural crest cells that migrate extensively before differentiating. (Exception is mouse embryo that ends gastrulation with ectoderm on the inside and endoderm on the outside and must undergo a process called turning to get the germ layers in the proper relationship. It does this at 8.5-9.6 days pf). 6 --the MESODERM is the middle layer and will produce muscle, connective tissue, bones, blood and blood vessels, kidneys, gonads --the ENDODERM is the inner layer and will produce the gut and internal organs such as liver, lungs, pancreas. SUPPLEMENTAL MATERIAL ON TIMING DURING DEVELOPMENT IN DIFFERENT MODEL ORGANISMS: S1. Let’s take advantage of this opportunity to go over the timing of events during embryogenesis in our model organisms, using gastrulation as the alignment point as suggested by O’Farrell et al. 1. The worm, C. elegans (Slide S1): a. shortest embryonic development in our group, the worm hatches out at 16 hr post-fertilization (pf) b. Gastrulation starts extremely early, at the 24-cell stage (about 90 min pf) c. Gastrulation is finished at about 6 hr, and the next 6-10 hr comprise organogenesis and elongation of the worm d. After hatching, the larva goes through 4 molts before becoming a sexually mature adult (with both sexes = hermaphrodite) at 72 hr pf 2. The fly, Drosophila (Slide S2): a. the fly takes about 24 hr to complete embryogenesis b. cleavage cycles precede gastrulation, which begins at 2.5-3 hr pf (we’ll go thru fly gastrulation in detail later) c. how long does fly gastrulation last? A long time – not complete until about 10 hr pf, so organogenesis begins during this time d. subsequent to gastrulation are several more dramatic movements, including a much studied process called dorsal closure e. upon hatching at 24 hr, the fly larva goes through 3 stages prior to pupation 3. The frog (Slide S3): a. most lab experiments are done with Xenopus, and timing is temp. dependent b. cleavage begins 3.5 hr pf, and gastrulation commences about 18 hr pf (stage 10) c. stages in frog very specific (we’ll go over later), mid-gastrula at stage 11, late gastrula at stage 12 7 d. the frog embryo hatches at about 36 hr pf 4. Zebrafish, Danio rerio (Slide S4): a. early development very fast, and most organ systems laid down by 24 hr pf, although the embryos don’t hatch until 48-72 hr pf b. cleavage stage embryos begin the mid-blastula transition at 2.75 hr pf, and the cells begin to move via EPIBOLY (defined above) at 4.66 hr pf c. however, gastrulation does not formally begin until 5.25 hr pf, and lasts until 10 hr pf. d. subsequent stages are segmentation (10.3-22 hr pf) where somites form, and pharyngula (24-42 hr pf) when heart starts to beat, pigmentation, and organogenesis 5. Chick (Slide S5): a. chick development is unique in that it begins before the egg is laid (24 hr pf), and in fact fertilization occurs before the shell is laid down. The newly laid chick egg has about 20,000 cells and is ready to begin gastrulation at 24 hr pf! b. total development takes 20-21 days to hatching in the chick c. gastrulation actually continues as somitogenesis (segmentation) and organogenesis begin (in the mouse too) because in chick and mouse there is an anterior-posterior gradient of development, so head and anterior end are further along than the tail! d. somitogenesis complete by 3.5-4 days pf, and much organogenesis occurs in the first third of development, later stages maturation and growth 6. The mouse, Mus musculus (Slide S6): a. total development is 19-20 days in the mouse b. as we already know, the “cleavage” divisions are very slow and the blastocyst at day 4.0 has only about 64-120 cells c. implantation occurs about day 4.5 pf, and after that the rapid divisions occur that O’Farrell et al call the true cleavage cycles. These divisions produce the cylinder stage embryo (day 5-6 pf) d. gastrulation in the mouse begins at about day 6.5 pf with the formation of the primitive streak that is the site of epiblast ingression to form endoderm and mesoderm. The epiblast that does not go through the streak becomes ectoderm e. again gastrulation continues for quite awhile in posterior regions of the embryo f. somitogenesis begins around day 7-7.5 pf 8 g. organogenesis peaks at day 9.5-13 or so. Optional reading: Reviews and commentaries: Heulsken J and Birchmeier W. (2001). New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet & Dev. 11, 547-553. Keller, R. (2005). Cell migration during gastrulation. Curr. Opin. Cell Biol. 17, 533-541. Leptin M and Affolter M. (2004). Drosophila gastrulation: identification of a missing link. Curr Biol 14, R480-R482. Mlodzik M. (2002). Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet 11, 564571. Benazeraf B and Pourquie O. (2007). Developmental biology: cell intercalation one step beyond. Curr Biology___, R119-R121. Articles: Winklbauer R, Medina A, Swain RK, and Steinbeisser H. (2001). Frizzled-7 signaling controls tissue separation during Xenopus gastrulation. Nature 413, 856-860. Davidson LA, Hoffstrom BG, Keller R, and DeSimone DW. (2002). Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha5 beta1, fibronectin, and tissue geometry. Dev Biol 242, 109-129. Yamamoto A, Amacher SL, Kin SH, Geissert D, Kimmel CB, and De Robertis EM. (1998). Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development 125, 3389-3397. 9 Habas R, Dawid IB, and He, X. (2003). Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes & Dev 17, 295-309. Medina A, Swain RK, Kuerner KM, Steinbeisser H. (2004). Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. EMBO J 23, 3249-3258. Gong Y, Mo C, Fraser SE. (2004). Planar cell polarity signaling controls cell division orientation during zebrafish gastrulation. Nature 430, 689-693. Nagel M, Tahinci E, Symes K, Winklbauer R. (2004). Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development 131, 2727-2736. Hukriede NA….Dawid IB. (2003). Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell 4, 83-94. Davidson LA, Marsden M, Keller R and DeSimone DW. (2006). Integrin 51 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol 16, 833-844. Gryzik T and Muller HJ. (2004). FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr Biol 14, 659-667. Stathopoulos A, Tam B… Levine M. (2004). pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev 18, 687-699. Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. (2007). The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449, 1049-1052. Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. (2008). Covergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135, 2435-2445. Wang Y, Janicki P, Koster I, Berger CD, Wenzl C, Grobhans J, Steinbeisser H. (2008). Xenopus paraxial protocadherin regulates morphogenesis by antagonizing sprouty. Genes & Dev. 22, 878-883. 10 11