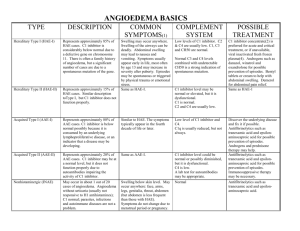
HAE type three - World Allergy Organization
advertisement

Global Guidelines for the Care of Patients with Hereditary Angioedema World Allergy Organization General Advisor WAO President Ruby Pawankar, Professor, Nippon Medical School, Tokyo General Advisor WAO President (Initiator of the guideline development): Richard Lockey, Professor of Medicine, University of South Florida Steering Committee Chair: Timothy Craig, Professor of Medicine and Pediatrics, Penn State University Members of the Steering committee: Emel Aygoren-Pursun, Professor of Medicine, University of Frankfurt Konrad Bork, Professor of Medicine, Johannes Gutenberg University Mainz Tom Bowen, Professor of Medicine, University of Calgary Henrik Boysen, Executive Director, HAEi-International Patient Organization Marco Cicardi, Professor of Medicine, University of Milan Henriette Farkas, Professor of Medicine, Semmelweis University Anete Grumach, Faculty of Medicine, University of Sao Paulo Connie Katelaris, Professor of Medicine, Allergy University of Western Syndney Hilary Longhurst, Consultant Immunologist, Barts and the London NHS Trust William R. Lumry, Clinical Professor of Medicine, University of Texas Southwestern Inmaculada Martinez-Saguer, Professor of Medicine, University of Frankfurt Marcus Maurer, Professor of Dermatology and Allergy, Charité – Universitätsmedizin Berlin Bruce Ritchie, Professor of Medicine, University of Alberta Bruce Zuraw, Professor of Medicine, University of California San Diego Pharmaceutical Supporters of the Guidelines in Alphabetical Order • • • • CSL Behring Dyax Shire Viropharma World Allergy Organization’s HAE Global Guidelines • Objectives: To develop a global approach for the management of patients with Hereditary Angioedema (HAE) so as to improve the quality of care delivered to patients with HAE • To increase the global availability of HAE medications • To encourage all physicians, patients, pharmaceutical companies and governments to ensure that patients with HAE are given similar access to appropriate therapies and care worldwide HAE Global Guidelines • Developed by a international group of physicians selected by the WAO who were considered HAE specialists • Evidenced based and rated • Supported by the literature • Reviewed by a larger international group of HAE experts • Approved by the WAO leadership • Finally, approved by WAO Member Allergy Associations HAE Global Guidelines Were developed to be: • A resource to clinicians for self education • Document to be a reference while providing clinic care • Teaching tool for physicians to have access to slides for educating others 1.0 Background and Introduction • • • • • 1.1 1.2 1.3 1.4 1.5 Terminology Genetics Pathophysiology Role of bradykinin Role of C-1-esterase 2.0 Presentation of HAE • 2.1 When to suspect HAE • 2.2 Triggers for HAE • 2.3 Prodromal symptoms 3.0 Diagnosing HAE • • • • 3.1 3.2 3.3 3.4 When to suspect HAE Diagnosing HAE Laboratory tests for HAE Flow chart for assessing angioedema 4.0 Suggested Future Terminology for Angioedema • Deferred to future conference 5.0 Treatment of HAE • 5.1 Acute treatment of attacks (On Demand) – – – – – – 5.1.1 5.1.2 5.1.3 5.1.4 5.1.5 5.1.6 C1-INH rcC1-INH FFP Icatibant Ecallantide Cost comparison 6.0 Short Term or Event Prophylaxis • • • • • • • • • • 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 Purpose of short term prophylaxis When to use it Therapies for Alternatives Therapies to avoid Dosing Androgens C1-INH Cost comparison References 7.0 Long Term Prophylaxis • • • • • • • • 7.0 Long term prophylaxis 7.1 Indications 7.2 Antifibrinolytics 7.3.1 Androgens 7.3.2 Children and chronic prophylaxis 7.3.3 Pregnant females and prophylaxis 7.3.4 Dosing for chronic prophylaxis 7.3.5 Adverse effects of androgens for chronic prophylaxis 7.0 Long Term Prophylaxis • • • • 7.3.6 7.4.1 7.4.2 7.4.3 Monitoring adverse effects of androgens C-1 Inhibitor- indications for chronic prophylaxis C1-inhibitor- efficacy Monitoring safety of C1-inhibitor 8.0 Preventive Medicine and Long Term Care of the Patient with HAE • • • • • • • • • 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 8.9 Trigger avoidance Drug avoidance Vaccine While using C1-INH While on androgens While on anti-fibrinolytics Family screening Summary table References 9.0 Caring for the Child with HAE • • • • • • • • • • 9.1 Why is the HAE child unique 9.2 When to test 9.3 Screening for HAE 9.4 Prenatal screening 9.5 Treatment of HAE 9.5.1 Dosing by weight is essential 9.5.2 Need for action plan 9.5.3 Dosing C1-INH for on demand 9.5.4 Prophylaxis 9.6 Pediatric references 10.0 Care of the Pregnant Patient with HAE • • • • • • • 10.1 10.2 10.3 10.4 10.5 10.6 10.7 Why pregnancy is unique Diagnosis of HAE during pregnancy Prenatal diagnosis In-vitro fertilization Abortion Lactation Family planning 10.0 Care of the pregnant patient with HAE • • • • • • • 10.8 Treatment during pregnancy 10.8.1 Long term prophylaxis therapy 10.8.2 Short term prophylaxis therapy 10.8.3 On demand therapy 10.9 Post partum care 10.10 Cost comparison in care 10.11 References 11.0 Special Issues in HAE • • • • • • • 11.1 11.2 11.3 11.4 11.5 11.6 11.7 Home and self therapy Patient support HAE associations Databases for HAE Action plans for HAE Need for better medications World wide access to therapies 1.1. As of now angioedema is known to be secondary to bradykinin and histamine. Histamine induced should be replaced by mast cell induced angioedema. (90% agreement) Mast cell-mediated angioedema: • • • • Allergic Idiopathic Autoimmune Drug induced Bradykinin - induced angioedema: • • • • • • ACE inhibitor induced Hereditary with low C1-INH (type-1) Hereditary with poorly functional C1-INH (type 2) Hereditary with normal C1-INH (type 3) Acquired with abnormal C1-INH Idiopathic C1INH Null Mice and Vascular Permeability C1INH gene B2BKR gene Evans blue +/+ +/+ No C1INH therapy No +/+ +/+ Yes No -/+/+ Yes No Adapted from Han ED, et al. J Clin Invest. 2002;109:1057-1063. -/+/+ Yes Yes -/-/Yes No In Vivo Generation of Kinins in HAE From Nussberger J, et al. J Allergy Clin Immunol. 1999;104:1321-1322. 1.2. Genetics of HAE Type I and II: • Autosomal dominant • Penetrance of type I and type II HAE is high • Large array of different mutations of the SERPING1 gene • De novo mutation of SERPING1 gene in 25% of cases Type III: • • • • Penetrance is variable A small subset of patients have a mutation in factor XII Genetic abnormality of most patients has not yet defined Requires a strong family history of angioedema since genetics is poorly defined. (90% consensus) The genetics of Type I and II Crowder JR, Crowder TR. Five generations of angioneurotic edema. Arch Inter Med 1917; 20:840-52 HAE Is Caused By C1 Inhibitor Mutations Bissler JJ, et al. Proc Assoc Am Physicians. 1997;109:164-173. Davis AE 3rd. Annu Rev Immunol. 1988;6:595-628. Verpy E, et al. Am J Hum Genet. 1996;59:308-319. Zuraw BL, Herschbach J. J Allergy Clin Immunol. 2000;105:541-546. C1-INH involved in 3 systems → C1-INH depletion Factor XIIa C1-INH Factor XII Contact System C1-INH Prekallikrein HMW-K Kallikrein Increased vascular permeability ANGIOEDEMA Complement System C1rs Plasminogen Bradykinin C1 Plasmin Fibrinolytic System C4 C2 1.3. Bradykinin is responsible for the angioedema associated with HAE • Plasma kallikrein and factor XII are normally inhibited by C1INH • Complement and contact plasma proteolytic cascades are activated with the potential to generate several vasoactive compounds. • Plasma kallikrein cleaves high molecular weight kininogen (HMWK) to generate bradykinin • Bradykinin is generated through activation of the contact system • Bradykinin is the primary mediator of swelling 1.4 C1-esterase inhibitor protein (C1INH) • Member of the serine protease inhibitor (serpin) superfamily • “Molecular mousetrap” • 1:1 stoichiometric relationship between the protease and C1INH • Major inhibitor of several complement proteases (C1r, C1s, and mannose-binding lectin–associated serine protease [MASP] 1 and 2) and contact-system proteases (plasma kallikrein and coagulation factor XIIa) • Relatively minor inhibitor of the fibrinolytic protease plasmin and the coagulation protease factor XIa. • C1-INH is deficient (type 1) or abnormal in function (type 2), but normal in type III HAE Active Cleaved: Inactive 1.5 Bradykinin • Nanopeptide generated by activation of the contact system. • Binds to the the B2 bradykinin receptor • Degradation product, des-Arg-bradykinin, binds to a separate B1 bradykinin receptor • Important for normal homeostasis, normal immune responses, inflammation, vascular tone, and vascular permeability. • Angioedema is primarily mediated through the B2 bradykinin receptor causing increase permeability • Increased vascular permeability is primarily mediated by down regulation of vascular endothelial cadherin and increased contraction of the actin cytoskeleton. Bradykinin Effect on Endothelial Cells Increased vascular permeability VE-cadherin Non stimulated Stimulated From Tiruppathi C, et al. Vascul Pharmacol. 2003;39:173-185. Actin stress fibers 2.0 Clinical Presentation of HAE • 2.1 When to suspect HAE • 2.2 Triggers for HAE • 2.3 Prodromal symptoms 2.1.1. Suspect HAE when a patient presents with angioedema, especially if free of urticaria, that is unpredictable in onset, but frequently follows a trigger such as trauma, and is associated with recurrent abdominal pain and upper airway swelling. (100% consensus) • • • • • Estimated prevalence of 1 per 50,000 All ethnicities and males and females equally effected Recurrent angioedema without urticaria 50% of the patients begin swelling before the age of 10 Recurrent swelling of the extremities, the gastrointestinal tract, the external genitourinary tract, the face, and the upper airway often (40%) proceeded by a trigger • Probability that a given attack will involve the skin or abdomen is nearly 50% each • Genitourinary and laryngeal attacks, account for only 3.6% of attacks 2.1.2. Continued • Swelling typically increases slowly over approximately 24 hours then resolving even more slowly over the subsequent 2 to 4 days • Preceded by prodromal symptoms, most classically erythema marginatum • Highly variable in individuals and within families. • Angioedema is often quite severe and unpredictable and limits productivity • Swelling of the upper airway can result in mortality • Abdominal swelling can cause nausea, vomiting and diarrhea and may result in inappropriate surgery Common triggers of HAE attacks Trauma Menstruation Angioedem Angioedema a attack Infection Medications: Estrogens ACE-I Stress 2.3 Prodromal Symptoms Frequently Proceed an Attack of HAE Prodromal symptoms include: • • • • • • • Flu-like feelings Erythema marginatum Parathesias Fatigue Indigestion Non-specific rashes Other non-specific symptoms The Percentage of Time That Patients Report Being Able to Predict an Acute HAE Attack Based on Prodromal Symptoms 100% of attacks 6.5% 8.7% Unable to predict 25% of attacks 26.1% 75% of attacks 50.0% 50% of attacks 8.7% Prematta M, et al. Allergy and Asthma Proceedings. 2009 Prodromal Symptom (%) Prodromal Symptoms That Patients Reported Before Their Last HAE Attack Percentage of Patients Prematta M, et al. Allergy and Asthma Proceedings. 2009 Erythema Marginatum before an HAE attack HAE Attacks can Involve • • • • • Face Extremities Upper airways Gastrointestinal system Genital-urinary system Intestinal swelling during an Abdominal attach 3 Diagnosing HAE • • • • 3.1 3.2 3.3 3.4 When to suspect HAE Diagnosing HAE Laboratory tests for HAE Flow chart for assessing angioedema 3.1. Patients with suspicion of HAE and family members of patients with HAE should be screened so that appropriate therapy can be available for treatment, especially since the first event may be of the upper airway and potentially may be fatal without appropriate therapy. (100% consensus) • In order to be prepared all patients with suspected HAE and all family members of patients diagnosed with HAE should be screened by at least a C4 • All patients with HAE should have a treatment action plan. • Patients may be asymptomatic even later in life; however, preparation is needed to have therapy available for procedures that can trigger HAE 3.3.1 Complement testing is used for the diagnoses, but is difficult to work with. As a result repeating the C4 and C1INH protein and function is important to confirm the diagnosis. (100% consensus) • C4- Is the best screening test for HAE and should be depressed in greater than 90% of patients even between attacks. A normal C4 during an attack virtually excludes the diagnosis of HAE 1 or 2 • C1-INH quantitative- low in type 1, normal Type II • C1-INH functional assay- Should be low in all type 1 and type 2, but the assay is not 100% specific nor sensitive. • C2- Often depressed during attacks. Not essential for the diagnosis • C1q- Should be normal in HAE. Indicated when patient presents with angioedema after the age of 40 years or if patient has constitutional symptoms • CH50- Indicated for complement deficiency, but not HAE 3.3.2. C4 is the single best screening test and repeating the C4 during an attack increases the probability that a low C4 will be found; however, C4 is not a definitive test since neither the sensitivity nor specificity are absolute. (100% consensus) • C4 level is an effective screening tool to detect C1INH deficiency • Occasional false negatives will be encountered, particularly in patients on anabolic androgens • Measurement of the activation product C4d may avoid false negative results. • False positives can also be encountered, especially in C4 deficiency. • C4 level is the best screening test, but alone cannot be relied upon to make a diagnosis of C1INH deficiency. 3.3.3 Other tests • Sequencing of the SERPING1 or factor XII genes can be done to pursue diagnoses of HAE type 1, 2, 3; however it is rare that this approach is needed. • Measurement of complement C3 levels OR CH50 is not useful. • Many patients with acquired C1INH deficiency have autoantibodies that recognize C1INH. 3.4 DIAGNOSING HAE Clinical symptoms + Familial history C4 Normal Low Confirm C4 during attack C1INH level Normal Low Normal Functional C1INH Consider HAE type III or AE due to medications C1q Normal Low Normal Low Consider other causes of C4 consumption HAE type II HAE type I AAE References for sections 1, 2 and 3 • • • • Agostoni A, Aygören-Pursun E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004 Sep. 1;114(3 Suppl):S51–131 Frank MM, Sergent JS, Kane MA, Alling DW. Epsilon aminocaproic acid therapy of hereditary angioneurotic edema: a double-blind study. N Engl J Med 1972; 286:808-12 • Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol: reversal of clinical and biochemical abnormalities. N Engl J Med 1976;295:1444-8 • Frank MM. Hereditary angioedema: the clinical syndrome and its management in the United States. Immunol Allergy Clin North Am 2006;26:653-68 • Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med 1972;287: 452-4 • Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol 1997;99:194-6 • Sze AL,Paki G, Varga L, Valentin S, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. J Allergy Clin Immunol 2005;115:864-9 • • • Sloane DE, Lee CW, Sheffer AL. Hereditary angioedema: safety of long-term stanozolol therapy. J Allergy Clin Immunol 2007;120:654-8 Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol 2008;100:153-61 • Bork K, Pitton M, Harten P, Koch P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet 1999;353:1066-7 • • • • • • Farkas H, Varga L, SzeÅLplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients.Pediatrics 2007;120(3):e713-e722 Cicardi M, Bergamaschini L, Tucci A, Agostoni A, Tornaghi G, Coggi G, et al. Morphologic evaluation of the liver in hereditary angioedema patients on long-term treatment with androgen derivatives. J Allergy Clin Immunol 1983;72:294-8 • • • Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol 1997;99:194-6 • • Zurlo JJ, Frank MM. The long-term safety of danazol in women with hereditary angioedema. Fertil Steril 1990;54:64-72 References for sections 1, 2 and 3 • Cicardi M, Bergamaschini L, Cugno M, Hack E, Agostoni G, Agostoni A. Long-term treatment of hereditary angioedema with attenuated androgens: a survey of a 13-year experience. J Allergy Clin Immunol 1991;87:768-73 • • • • Lundh B, Laurell AB, Wetterqvist H, White T, Granerus G. A case of hereditary angioneurotic oedema, successfully treated with epsilon aminocaproic acid: studies on C’1 esterase inhibitor, C’1 activation, plasminogen level and histamine metabolism. Clin Exp Immunol 1968; 3:733-45 • • • • • • • • • • Bork K, Witzke G. Long-term prophylaxis with C1-inhibitor (C1 INH) concentrate in patients with recurrent angioedema caused by hereditary and acquired C1-inhibitor deficiency. J Allergy Clin Immunol 1989;83: 677-82 • • • • • • • • • • • • • • • • • Royston D. Blood-Sparing Drugs: Aprotinin, Tranexamic Acid, and Epsilon-Aminocaproic Acid. Int Anesthesiol Clin 1995; 33(1):155-7. Parish LC. Hereditary angioedema: Diagnosis and management-a perspective for the dermatologist. J Am Acad Dermatol. 2011 May 5. [Epub ahead of print] Riedl M, Gower RG, Chrvala CA. Current medical management of hereditary angioedema: results from a large survey of US physicians. Ann Allergy Asthma Immunol. 2011 Apr;106(4):316-322.e4. Epub 2011 Feb 5. Varga L, Füst G, Csuka D, Farkas H. Treatment with C1-inhibitor concentrate does not induce IgM type anti-C1 inhibitor antibodies in patients with hereditary angioedema. Mol Immunol. 2011 Jan;48(4):572-6. Epub 2010 Dec 15. Tallroth GA. Long-term prophylaxis of hereditary angioedema with a pasteurized C1 inhibitor concentrate. Int Arch Allergy Immunol. 2011;154(4):356-9. Epub 2010 Oct 26. Maurer M, Magerl M. Long-term prophylaxis of hereditary angioedema with androgen derivates: a critical appraisal and potential alternatives. J Dtsch Dermatol Ges. 2011 Feb;9(2):99-107. doi: 10.1111/j.1610-0387.2010.07546.x. Epub 2010 Oct 15. Bork K, Hardt J. Hereditary angioedema: long-term treatment with one or more injections of C1 inhibitor concentrate per week. Int Arch Allergy Immunol. 2011;154(1):818. Ebo DG, Verweij MM, De Knop KJ, Hagendorens MM, Bridts CH, De Clerck LS, Stevens WJ. Hereditary angioedema in childhood: an approach to management. Paediatr Drugs. 2010 Aug 1;12(4):257-68. Czaller I, Visy B, Csuka D, Füst G, Tóth F, Farkas H. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: a long-term survey. Eur J Obstet Gynecol Reprod Biol. 2010 Sep;152(1):44-9. Epub 2010 Jun 11. Farkas H, Czaller I, Csuka D, Vas A, Valentin S, Varga L, Széplaki G, Jakab L, Füst G, Prohászka Z, Harmat G, Visy B, Karádi I. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema-a longitudinal study. Eur J Clin Pharmacol. 2010 Apr;66(4):419-26. Epub 2009 Dec 19. Farkas H. Pediatric hereditary angioedema due to C1-inhibitor deficiency. Allergy Asthma Clin Immunol. 2010 Jul 28;6(1):18. Zanichelli A, Vacchini R, Badini M, Penna V, Cicardi M. Standard care impact on angioedema because of hereditary C1 inhibitor deficiency: a 21-month prospective study in a cohort of 103 patients. Allergy. 2011 Feb;66(2):192-6. Because of the differential diagnosis it is important to determine the exact diagnosis so that appropriate therapy for the specific type of angioedema the patient is manifesting can be utilized. (100%) Bradykinin Induced • HAE type 1 (C1-INH deficiency), • HAE type 2 (C1INH dysfunctional), • HAE type 3 (HAE with normal C1INH), • AAE (acquired C1INH deficiency), • ACE-I induced, idiopathic bradykinin induced angioedema, Histamine Induced (Mast cell dependent) • Idiopathic histamine induced angioedema • Allergic angioedema • Drug induced histamine dependent angioedema 3.0 Bradykiniin-induced Angioedema Differential Frequency Age of first symptoms Pathogenesis Clinical Manifestations Treatment Angioedema due to ACE inhibitors 0.1 – 0.5% of the patients treating with ACE inhibitors Any period after drug introduction Disturbance of bradykinin metabolism Facial subcutaneous and upper airway edema (rarely gastrointestinal) Drug removal HAE 1:10000 - 1:50000 inhabitants childhood Functional or quantitative C1INH Subcutaneous, upper airway and gastrointestinal edema androgens, plasmin inhibitors, C1-INH, icatibant and ecallantide Suspect Mast cell Dependent when • Urticaria coexists with angioedmea • Majority of cases (Histamine induced disease is far more common than bradykinin induced so trial of antihistamines and corticosteroids is indicated until diagnosis is confirmed). • Response to antihistamines • Obvious trigger (i.e. drug, food) Suspect Acquired Angioedema • Angioedema similar to HAE, but later onset (over 40 years) • Constitutional symptoms • Low C1q • Lymphoma or monoclonal gammopathy • Anti-C1INH antibody Hereditary Angioedema with Normal C1 Inhibitor (HAE type III) Background: • In 2000, report on ten families and a further report on one family with HAE and normal activity of C1-INH in plasma. All 43 patients were women • In 2006, report of the first men with HAE type III • In 2006, report of 2 different mutations in exon 9 of the coagulation factor XII gene (missense mutations) • In 2006, report that the mutations cause an increased factor XII activity (gain of function mutation) • In 2009, investigation in which no increased factor XII activity in the plasma of patients with HAE-FXII could be found Hereditary Angioedema with Normal C1 Inhibitor (HAE type III) Diagnosis Clinical Presentation: • Appearance of first clinical symptoms mostly in the second decade of life • Relapsing swellings of various organs: skin swellings, abdominal pain attacks, tongue swellings, laryngeal edema, pharyngeal edema • In some women, the onset of symptoms occurs during pregnancy or after starting estrogen-containing oral contraceptives or hormonal replacement therapy • In other women, the symptoms occur exclusively during pregnancy, the intake of oral contraceptives or hormonal replacement therapy; however, in others symptoms occur independently from these time periods Hereditary Angioedema with Normal C1 Inhibitor (HAE type III) Assessment and Work Up: • The diagnosis of “HAE type III” is based on the typical clinical symptoms in combination with the familial occurrence • There is no laboratory that can confirm the diagnosis of “HAE type III” • A search for one of the mutations in the factor XII gene may serve to differentiate “Hereditary angioedema with normal C1 inhibitor with a mutation in the factor XII gene (HAE-FXII)” from “Hereditary angioedema with normal C1 inhibitor without a mutation in the factor XII gene (HAE-unknown), but a negative test does not exclude HAE Hereditary Angioedema with Normal C1 Inhibitor (HAE type III) Treatment: • A limited number of patients with HAE type III have been reported • Experience with treatments is minimal • Treatments used for HAE type III attacks include C1 inhibitor concentrate, ecallantide and icatibant and for prophylaxis androgens, tranexamic acid, and progesterone Hereditary Angioedema with Normal C1 Inhibitor (HAE type III) Prognosis: • The frequency of acute attacks varies considerably from patient to patient • There are many patients with a low clinical disease expression • Cases of asphyxiation have been described 4.0 Suggested Terminology for Angioedema for the Future Will address this in a future meeting References • • • • • • • • Cicardi M. Zanichelli A. The acquired deficiency of C1-inhibitor: lymphoproliferation and angioedema. Current Molecular Medicine. 10(4):354-60, 2010 Jun. UI: 20455857 Agostoni A. Aygoren-Pursun E. Binkley KE. Blanch A. Bork K. Bouillet L. Bucher C. Castaldo AJ. Cicardi M. Davis AE. De Carolis C. Drouet C. Duponchel C. Farkas H. Fay K. Fekete B. Fischer B. Fontana L. Fust G. Giacomelli R. Groner A. Hack CE. Harmat G. Jakenfelds J. Juers M. Kalmar L. Kaposi PN. Karadi I. Kitzinger A. Kollar T. Kreuz W. Lakatos P. Longhurst HJ. Lopez-Trascasa M. Martinez-Saguer I. Monnier N. Nagy I. Nemeth E. Nielsen EW. Nuijens JH. O'grady C. Pappalardo E. Penna V. Perricone C. Perricone R. Rauch U. Roche O. Rusicke E. Spath PJ. Szendei G. Takacs E. Tordai A. Truedsson L. Varga L. Visy B. Williams K. Zanichelli A. Zingale L. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. Journal of Allergy & Clinical Immunology. 114(3 Suppl):S51131, 2004 Sep. UI: 15356535 D'Incan M. Tridon A. Ponard D. Dumestre-Perard C. Ferrier-Le Bouedec M. Betail G. Souteyrand P. Caillaud D. Acquired angioedema with C1 inhibitor deficiency: is the distinction between type I and type II still relevant? Dermatology. 199(3):227-30, 1999. UI: 10592402 Bork K. Barnstedt SE. Koch P. Traupe H. Bork K. Barnstedt SE. Koch P. Traupe H. Lancet. 356(9225):213-7, 2000 Jul 15. UI: 10963200 5.0 Treatment of HAE • 5.1 Acute treatment of attacks (On Demand) – – – – – 5.1.1- C1-INH 5.1.2- rcC1-INH 5.1.3- FFP 5.1.4- Icatibant 5.1.5- Ecallantide • 5.2 Short Term Prophylaxis (pre-procedural) • 5.3 Long Term Prophylaxis (Suppression of attacks) 5.1 On Demand Therapies • • • • • Human plasma-derived C1-esterase inhibitor (hpC1-INH) Recombinant C1-INH (rcC1-INH) Ecallantide Icatibant Fresh Frozen Plasma 5.1.1 Treatment with C1-INH made from purified human blood eliminates the underlying cause of HAE by replacing the deficient protein. (100%) Its mode of action is physiological: • It is an endogenous plasma factor, • Acting on the same targets as patient’s own C1-INH, it inhibits all cascade systems involved in the production of bradykinin released during attacks. References for sections On Demand Therapy with C1-INH • • • • Zuraw BL.Hereditary angioedema. N Engl J Med 2008;359:1027-36. Cicardi M, Theuse of plasma-derived C1 inhibitor in the treatment of hereditary angioedema. Expert Opin Pharmacother. 2007 Dec;8(18):3173-81. Joseph K, Tholanikunnel TE, Kaplan AP. Treatment of episodes of hereditary angioedema with C1 inhibitor: serial assessment of observed abnormalities of the plasma bradykinin forming pathway and fibrinolysis. Ann Allergy Asthma Immunol. 2010 Jan;104(1):50-4. AE Davis, 3rd, F Lu, P Mejia. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost 2010; 104(5). Medicinal Products Human Plasma-Derived C1-INH Concentrate • Berinert® P (CSL Behring, Marburg, Germany) – pasteurization, specific chromatography, nanofiltered – plasma half-life: 32 to 47 hours – marketing authorization in 1985; licensed in 2009 in the USA • Cetor ® (Sanquin, T Amsterdam, The Netherlands) – precipitation, pasteurization, nanofiltration, – plasma half-life: 48±10 hours – licensed in 1997 in Europe • Cinryze® (Viropharma, USA) – licensed currently for prophylaxis in USA and prophylaxis and on demand therapy in Europe Medicinal Products Recombinant C1-INH Concentrate • Ruconest (Pharming, The Netherlands), – manufactured from the breast milk of transgenic rabbits – plasma half-life: approx. 3 hours – Licensed in EU for on demand therapy Indications of C1-INH are partially off label and all plasma derived products can be used in place of each other (100%) Berinert® all types of attacks in adults, children, pregnant women also during breastfeeding. Dose is 20units/kg Cinryze ® all types of attacks in adults, children, pregnant women also during breastfeeding. Dose is 1000 units Cetor ® all types of attacks in adults, children, pregnant women also during breastfeeding. Dose is 1000 units Berinert® P • 20 U/kg body weight (previous, non-placebo-controlled studies demonstrated the efficacy of doses of 500 to 1000 U/attack.) • Until further double-blinded data are available 20 units per kg is the suggested dose for on demand therapy of C1INH (vote 90%) Results Craig et al. JACI 2010 Pasteurized Plasma C1-INH Concentrate (Berinert) Pasteurized C1-INH Bernstein JA, et al. J Allergy Clin Immunol. 2008;121[Abstract LB16]. Cetor® • 1000 U should be administered at the first sign of the onset of an acute attack. Then, if the patient has not responded adequately after 60 minutes, a second 1000 U dose can be given. • Previous, non-placebo-controlled studies demonstrated the efficacy of doses of 500 to 1000 U/attack. (vote-90%) C1-INH Method of Administration • intravenously, by slow (1 mL/min) injection or infusion, by medical professional in the home or hospital setting, or for self-administration • storage: 2 to 25 °C- expiration varies by product • a single, glass vial of 500 U C1-INH concentrate lympholized powder should be reconstituted with 5 to 10 mL sterile water for injection – it does not contain any preservative • Approved dosage: Berinert® P: 20 U/kg body weight Cinryze and Cetor ® : 1000 U/attack When to treat Subcutaneous Attacks Recommended: • Edema of the extremities: if the attack is severe, edema is extensive, associated with significant pain, or functional limitations or compromise of blood circulation the person should be treated. • Edema of the neck and face, as this can propagate to the upper airways • Edema of the trunk, as this can cause breathing difficulty • Edema of the genitals, as this can cause micturition problems May be administered: • To relieve mild edema of the extremities, when the latter reduces working capacity or interferes with activities of daily living Method of Administration UPPER AIRWAY EDEMA • in every case – as early as upon the onset of initial symptoms – in order to prevent progression to a life-threatening condition • the patient should be monitored until the resolution of symptoms is complete, at a facility where preconditions for establishing and maintaining airway patency are guaranteed Sore, scratchy, itchy throat ‘Something has stuck in my throat’ Lump sensation in the throat Feeling of tightness Dysphagia Voice changes High-pitched or hoarse voice Roughness of voice Resonant, ‘barky’ cough Stridor Dyspnea Fear of suffocation Aphonia Inability to breathe, speak or cough Anxiety and agitation INITIAL SYMPTOMS (edema on face and neck) Use of C1-INH in HAE Recommendation: • • • • The risk versus benefits of C1INH favors benefits. Few adverse events have been reported. All 3 products are equal in efficacy and adverse effect profile. The risk is minimal, but absolute safety cannot be assumed since it is a human blood product. (100%) References for C1-INH • • • • • Waytes Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med, (1996), 334(25): 1630-4., Kunschak, M; Engl, W; Maritsch, F, Et Al.: A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion, (1998), 38(6): 540-9. Craig TJ, Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009 Oct;124(4): Cicardi, M; Hereditary angioedema: an appraisal of 104 cases. Am J Med Sci, (1982), 284(1): 29. Agostoni, A; Cicardi, M: Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore), (1992), 71(4): 206-15. Bork, K; Barnstedt, S E: Treatment of 193 Episodes of Laryngeal Edema With C1 Inhibitor Concentrate in Patients With Hereditary Angioedema. Arch Intern Med, (2001), 161(5): 714-718. Adverse Effects Allergic Reaction: • Risk is negligible and has usually resulted from inappropriate administration (e.g. too rapid infusion of cold solution), or poorly mixed product. • Recommendations: Dosage instructions should be observed. When a hypersensitivity reaction occurs, patient data and the batch number of the preparation should be recorded to document a relationship. (100%) • No reports of transmission of infections or viral pathogens. Post-marketing experience with Berinert® P (1985-2003) demonstrates that neither approved, nor off-label use is associated with transmission of HBV, HCV or HIV-1/-2. (Jurs) Recommendations: hepatitis A and B vaccine of HAE patients expected to receive hp-C1INH(100%) Adverse Events • Antibody formation against to C1-INH protein has been documented, but without loss of function • Overdose Thrombotic events have occurred in patients receiving high doses of Berinert off-label (i.e. to relieve capillary leak syndrome, as well as before, during, or after heart surgery during the period of extracorporeal circulation) • Interaction with other medicinal products and other forms of interaction None known. • Tachyphylaxis Does not occur (Frequent and repetitive use may increase attack frequency and severity, but does not compromise the effectiveness of the drug.) ADVANTAGES of C1-INH DRAWBACKS • A natural product • Inhibits all cascade systems involved in the generation of bradykinin • Its half-life is longer than those of other drugs • it rapidly reaches peak plasma concentration • Rapid onset of action • Lack of rebound angioedema • Effective in all types of attacks • Safe for children • Safe for pregnant women • Safe for home use • Minimal allergic reaction • No tachyphylaxis • Long (over 30 years) clinical experience • Presently widely available, compared to other drugs • Expensive (but its price is not higher than those of other drugs for AT) • Potential risk for the transmission of other diseases and infective agents • Intravenous administration • Frequent and repetitive use may influence attack frequency and severity • Off label administration of high doses is associated with thrombotic events C1-INH adverse event references • • • • • Zingale, L C; Pappalardo, E; Zanichelli, A, Et Al.: C1 inhibitor concentrate: efficacy and adverse reactions. Int. Immunopharmacol., (2002), 318(1385 (abs). De Serres J, Groner A, Linder J. Safety and efficacy of pasteurized C1-inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apheresis Sci. 2003;29:247–254. Craig TJ, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz D, Obtułowicz K, Reshef A, Ritchie B, Moldovan D, Shirov T, Grivcheva-Panovska V, Kiessling PC, Keinecke HO, Bernstein JA. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009 Oct;124 Farkas H, Jakab L, Temesszentandrási G, Visy B, Harmat G, Füst G, Széplaki G, Fekete B, Karádi I, Varga L. Hereditary angioedema: a decade of human C1-inhibitor concentrate therapy. J Allergy Clin Immunol. 2007 Oct;120(4):941-7. Bork K, Hardt J. Hereditary angioedema: increased number of attacks after frequent treatments with C1 inhibitor concentrate. Am J Med. 2009 Aug;122(8):780-3. Recommendation: For on demand therapy rcC1-INH appears to be equally effective to plasma derived C1-INH (no vote) • The mode of action of recombinant human C1-INH (rhC1-INH) is identical to that of pdC1INH concentrate. • Administration of a fixed dose of 2100 units restored functional C1-INH level within the normal range in approximately 75% of patients. • Freeze-dried protein, 99% pure, free of preservatives. • Human plasma protease C1 inhibitor, glycoform-α, N,Oglycosylated recombinant protein secreted in rabbit milk • Ruconest/Rhucin 1 unit corresponds to the mean quantity of C1-INH present in 1 ml fresh, normal plasma 5.1.3 Recommendation: For on demand therapy C1-INH appears to be equally effective to plasma derived C1-INH Differences between rcC1-INH and C1-INH • • • • rcC1-INH half-life is 3 hours Contains traces of rabbit antigen Contraindicated in rabbit allergy Unique polysaccharides are added to the protein during production in the rabbit • No human blood-borne disease associated with it • Supply potentially endless Recommendation: Patients with rabbit allergy should avoid the use of rcC1INH (100%) • Ruconest is indicated for the treatment of all types of HAE attacks in adults. • Ruconest should not be used in individuals that may be hypersensitive (allergic) to conestat-alfa or any of the other ingredients. • It must not be used in patients with a known or suspected allergy to rabbit dander. • If rabbit allergy in question, immunocap or percutaneous skin test should be performed before administration Recommendation: The recommended dose for the routine treatment of acute attacks is 50 U/kg body weight (100%) • • • • • Test for rabbit antibodies before using the rcC1-INH Conestat-alfa contains 2100 units per vial concentration of 150 units/ml treatment of acute attacks is 50 U/kg body weight maximum of 4200 U (2 vials) for patients of or over 84kg body weight • second injection may be given if the patient does not improve satisfactorily after the first dose Efficacy of Recombinant Human C1-INH n=13 n=13 Placebo Placebo 50 u 100 u Pharming Group NV. www.pharming.com/index.php?act=medi. 50 u 100u References for rcC1-INH • RA Hack CE, Haase G, Pijpstra R. Mechanistic implications from the treatment of acute angioedema attacks in Hereditary Angioedema (HAE) with recombinant human C1 inhibitor (rhC1INH) (poster). XXIII International Complement Workshop 1-5 Aug 2010, New York. • A Relan, G Haase, E Hack, J Nuijens, B Giannetti, R Pijpstra. Dose justification for recombinant human C1INH for the treatment of acute angioedema attacks in patients with hereditary angioedema. Allergy 2010; 65(s92):S1186. AE Davis, 3rd, F Lu, P Mejia. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost 2010; 104(5). ADVANTAGES of rcC1-INH • Inhibits all cascade systems involved in the generation of bradykinin • Rapid onset of action • Lack of rebound angioedema • Effective in all types of attacks • Safe for children • Safe for pregnant women • Minimal allergic reaction • No tachyphylaxis • No viral transmission • Unlimited supply DRAWBACKS • Expensive (but its price is not higher than those of other drugs for AT) • Potential risk for anaphylaxis • Intravenous administration • Potential, but not described, for neutralizing antibodies and IgE to polysaccharide added to the protein 5.1.3 Recommendation: Fresh Frozen Plasma should only be used for on-demand therapy when other medications are not available (100%) • action- replaces C1-INH • indication- not indicated unless no other treatments are available • method- 2 units for adults, weight based for children • adverse events – anaphylaxis, worsening of HAE, viral transmission • positives and negatives- negatives out-weigh positives and FFP should be avoided if other therapies are available • Always use solvent treated virus inactivated product when available 5.1.4 Treatment of HAE: Icatibant • Recommendation: Icatibant is effective for treatment of HAE attacks at all locations with a dose of 30 mg SQ (100%) • Recommendation: Repeat dosing of icatibant is sometimes necessary and up to10% require a second dose and 1% a third dose to treat an HAE attack (not voted) 5.1.4 Treatment of HAE: Icatibant Mechanism C1q Complement system ‘Contact’ system Immune complex (AgAb) C1 I Negatively charged surface Factor X11 Factor X11a NH C1qrs C1INH • Small 10 AA peptide • Competitive inhibitor of bradykinin B2 receptor Factor X11f C4, C2 and classical pathway activation C1INH Prekallikrein Kallikrein C1INH High molecular weight kininogen Bradykinin Bradykinin-1 receptor binding KininaseI Aminopeptidase Pain Des Arg Bradykinin Bradykinin-2 receptor binding Angiotensin converting enzyme (ACE) Vasodilation Increased vascular permeability Inactive metabolites 5.1.4 Treatment of HAE: Icatibant Indication • Treatment of acute HAE • Any site • Not licensed for adolescents, children or pregnant/lactating women due to lack of safety data in these populations 5.1.4 Treatment of HAE: Icatibant – Trial Outcomes Cicardi et al NEJM 2010 5.1.4 Treatment of HAE: Icatibant – Adverse Events • Local erythema, swelling and pain at injection site – almost every patient (97%) • Systemic events comparable with placebo • Recurrent swellings – 10% in FAST I&II open label extension Positives • Ease of Use • Rapid to Administer • Appear to have good safety profile •Not immunogenic • Alternative to C1 inhibitor • Ideal for self administration • May address problem of delays in accessing treatment • Not Intravenous Negatives • Short half life-probably not suitable for prophylaxis • Need for repeat dosing • Not currently recommended for pregnant women or children • No action on other systems regulated by C1 inhibitor • Pain and burning at injection site Cicardi et al NEJM 2010 5.1.5 Treatment of HAE: Ecallantide • Recommendation: Ecallantide at 30 mg SQ can be used to treat HAE attacks at all locations (100%) in patients aged 16 and above • Recommendation: Self injection of ecallantide should be avoided secondary to a small, but real risk of anaphylaxis (100%) Ecallantide (Kalbitor) • Manufactured by DYAX • Highly selective, potent, reversible inhibitor of plasma kallekrein • 60 amino acid peptide derived from a Kunitz domain backbone with 7 unique amino acids. • Kallekrein on rate: 2x106M-1s-1 • off rate 2x10-5s-1 • Can be administered IV or SQ (30 mg SQ) Effect of Ecallantide on Treatment Outcome Score (TOS) US Food and Drug Administration. www.fda.gov/ohrms/dockets/AC/09/briefing/2009-4413b1-03Dyax.pdf. Effect of Ecallantide on MSCS in HAE Attacks placebo P=0.01 DX88 MSCS Score US Food and Drug Administration. www.fda.gov/ohrms/dockets/AC/09/briefing/2009-4413b1-03Dyax.pdf. Ecallantide Safety • Ecallantide is generally well tolerated • Most events were mild; similar to placebo • Related adverse events – Gastrointestinal (diarrhea, abdominal pain, nausea) – Upper respiratory infections (colds, cough, pharyngitis) – Headache, fatigue – Abnormal results in tests of coagulation • Known effect on activated partial thromboplastin time • No patients with clinically significant bleeding – Anaphylactic/anaphylactoid reactions in some patients • Role of contaminating Pichia proteins • Some patients develop anti-drug antibodies (IgG and IgE) • True anaphylactic reaction on rechallenge has occurred 5.1.5 Treatment of HAE: Ecallantide • Hypersensitivity reactions occur in approximately 3% of patients that receive ecallantide. • IgG and IgE antibodies are produced against ecallantide, but the IgG does not appear to be neutralizing. • Approved in the USA to be giving at home by a health care provider equipped and trained to treat anaphylaxis How Do the Newer Drugs Compare? Drug Advantages Disadvantages Best use Status Plasmaderived C1-INH • Extensive clinical experience • Corrects the fundamental defect • long half-life • Infectious risk • Needs IV access • Limited supply • Acute attacks • Short-term • Long-term prophylaxis • Prodromes • Berinert P: approved for attacks • Cinryze: approved for prophylaxis and attacks • Cetor approved for attacks Recombinant C1-INH • Corrects the fundamental defect • No human virus risk • Scalable supply • Needs IV access • Short half-life • Potential for allergic reactions • Acute attacks • Short prophylaxis? • Prodrome? • Rhucin: used for attacks Ecallantide • No infectious risk • Subcutaneous administration • Antibodies may cause allergic reaction or neutralization • Short half-life • Acute attacks in office or by HCP in home • Kalbitor approved for administration by HCP for attacks Icatibant • No infectious risk • Stable at room temperature • Subcutaneous • Short half-life • Local pain or irritation • Self treatment of acute attacks • Firazyr: used for attacks 6.0 Short-term or Pre-Procedural Therapy • 6.1 Short term prophylaxis should be considered to cover periods of high risk for attacks when there is either: – increased likelihood of attack – increased consequence of attack • [100% consensus. Observational/ case series] 6.1 Short-term or pre-procedural therapy: Indication • Prior to some surgeries; especially – dental/ intraoral surgery – where intubation is required – major surgery • To cover periods of high risk for attacks or very important events – increased likelihood of attack – increased consequence of attack Evidence limited to case reports, small series and one controlled study (Bork 2011) – recommendation based on expert opinion. 6.2 Treatments for short term prophylaxis • For short term prophylaxis, the following may be used (best option depends on patient and surgical factors and should be decided by physician) – C1 inhibitor (plasma-derived) – Attenuated androgens (danazol, stanozolol, or oxandrolone) • [100% agreement. Observational/ case series] 6.3 Short term prophylaxis for lower risk procedures • For lower risk procedures, or where safe prophylactic agents are not available, prophylaxis may be omitted • the patient should be aware of the risk of and have a management plan for attacks, which are more likely to occur after surgery. – Two doses of C1 inhibitor, ecallantide or icatibant should be immediately available. • [100% agreement. Observational/ case series] 6.3 Short-term or pre-procedural therapy: on demand in place of short term prophylaxis • • • • Increased risk of swelling 4- 30 hours after surgery Swellings occur despite prophylaxis Absolute risk is low Swelling usually near site of trauma Decision to treat with prophylaxis depends on patient & surgical factors A plan to treat attacks is necessary despite prophylaxis since breakthrough can occur 6. 4 Therapies not to be used for short term prophylaxis • The following are not recommended: – Plasma (FFP/SDP)*. (only if no other therapies are available) – Icatibant – Ecallantide – Methyl testosterone • Probably effective. – Ruconest/ Rhucin • [100% agreement except for plasma (75% agreement). Expert opinion] 6.5 Dosing for short term prophylaxis • Recommended doses/timings are as follows – C1 inhibitor (plasma-derived) is a preferred drug for prophylaxis and should be used as close to the event as possible at a dose of 20 U/kg or a fixed dose of 1000 units – Danazol 2.5-10mg/kg//day (for adults or children), maximum 600 mg* a day or Stanozolol 4-6 mg* a day for 5 days before and 2 days after procedure • [75% agreement. Expert opinion] 6.6 Short-term or pre-procedural therapy: Attenuated Androgens - REGIMEN Document Recommendation Alternative Children Duration UK Consensus 2005 Danazol 200600 mg Stanozolol 2-6 mg od Danazol 300mg od 5 days before, 2 days after procedure International (Canadian/ Hungarian) Consensus 2010 Danazol 2.510mg/kg//day: max 600 mg / Stanozolol 4-6 mg od Farkas 2010 Danazol 600mg Doses are expert opinion: not evidence-based 5 days before, 2 days after procedure 4 days before and after procedure 6.6 Short-term or pre-procedural therapy: Attenuated Androgens - TOXICITY • menstrual disturbance, • vaginal dryness or irritation • emotional labiality [Danazol USP] • Blood tests or other safety monitoring is not necessary [Birjmohun R 2008]. 6.6 Androgens as short term prophylaxis Advantages Disadvantages Ease of use Perceived inferior efficacy to C1 inhibitor Well tolerated in short term (usually) Need to start several days prior to procedure Concern of side effects, but minimal Have been used in children without problem For children dosage based on weight Have been used in pregnancy (3rd trimester) without problem Not suitable for most pregnant women and during breast feeding Low cost Unavailable in some countries Availability 6.7 C1-inhibitor for short term prophylaxis • There are few reports about the efficacy of a preoperative short-term prophylaxis with C1-INH concentrate (observational data) (6;17-21). • Intravenous injection one hour before surgery ensures a sufficiently high plasma level of C1-INH during critical time during and after surgery. • On demand therapy for HAE swelling may still be required. 6.7 Dosing of C1-inhibitor for short term prophylaxis • Retrospective study on 171 patients with 705 sessions with extraction of one tooth or more teeth.[9] Without prophylaxis 124 HAE attacks followed 577 (21.5%) tooth extractions. With a pre-operative prophylaxis with 500 U C1-INH concentrate 12/75 (16%) tooth extractions were followed by HAE attacks and with a prophylaxis with 1000 U 4/53 (7.5%). The graded dose-response relationship was significant at p <0.05. All attacks were either facial or upper airway swellings or a combination of both. • From this best dose is probably between 10 and 20 units per kg Bork et al 2011 6.7 Safety of C1-inhibitor for short term prophylaxis Safety: • C1-INH concentrates are well tolerated in the treatment of attacks of HAE(12) and also when used for preoperative prophylaxis (24) 6.7 Advantages and disadvantages for C1-inhibitor for short term prophylaxis Advantages Disadvantages Some evidence for efficacy Good theoretical rationale for use Intravenous Well tolerated Lack of availability in some countries Treatment of choice in children and pregnancy Cost May give additional doses if swellings occur 6.8 Cost comparison of drugs for short term prophylaxis • Drug Cost in USD • • • • • • +++++ +++++ + + (not recommended) variable (cost of on demand) ++ Berinert (20 u/kg) Cinryze (1000 units) Androgens Tranexamic acid No prophylaxis FFP 6.9 Short term prophylaxis references • • • • • • • • (1) Degroote DF, Smith GL, Huttula GS. Acute airway obstruction following tooth extraction in hereditary angioedema. J Oral Maxillofac Surg 1985 January;43(1):52-4. (2) Bork K, Barnstedt S-E. Laryngeal edema and death by asphyxiation following tooth extraction in four patients with hereditary angioedema. Am Dent Assoc 2003;134(8):108894. (3) Lodi G, Sardella A, Bez C, Demarosi F, Cicardi M, Carrassi A. Dental experience and self-perceived dental care needs of patients with angioedema. Spec Care Dentist 2001;21(1):27-31. (4) Bork K, Hardt J, Staubach-Renz P, Witzke G. Risk of laryngeal edema and facial swellings after tooth extraction in patients with hereditary angioedema with and without prophylaxis with C1 inhibitor concentrate: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011 July;112(1):58-64. (5) Maeda S, Miyawaki T, Nomura S, Yagi T, Shimada M. Management of oral surgery in patients with hereditary or acquired angioedemas: review and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003 November;96(5):540-3. (6) Maves KK, Weiler JM. Tonsillectomy in a patient with hereditary angioedema after prophylaxis with C1 inhibitor concentrate. Ann Allergy 1994 November;73(5):435-8. (7) Malmstrom HS, Hock JM, Sanford C. Dental management of patients with hereditary angioedema: report of case. J Am Dent Assoc 1985 December;111(6):957-8. (8) Bouillet L, Longhurst H, Boccon-Gibod I, Bork K, Bucher C, Bygum A et al. Disease expression in women with hereditary angioedema. American Journal of Obsterics and Gynaecology 2008;199(5):484. Short term prophylaxis references • • • • • • • • (9) Farkas H. Pediatric hereditary angioedema due to C1-inhibitor deficiency. Allergy Asthma Clin Immunol 2010 July 28;6(1):18. (10) Farkas H, Gyeney L, Gidofalvy E, Fust G, Varga L. The efficacy of short-term danazol prophylaxis in hereditary angioedema patients undergoing maxillofacial and dental procedures. Journal of Oral and Maxillofacial Surgery 57[4], 404-408. 1999. Ref Type: Abstract (11) Atkinson JC, Frank MM. Dental management of hereditary angioedema. Journal of Oral Pathology and Medicine 20, 139-142. 1991. Ref Type: Abstract (12) Marques L, Domingo D, Maravall FJ, Clotet J. Short-term prophylactic treatment of hereditary angioedema with icatibant. Allergy 2010 January;65(1):137-8. (13) Bowen T, Cicardi M, Farkas H, Bork K, Longhurst HJ, Zuraw B et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol 2010 July 28;6(1):24. (14) Sheffer AL, Fearon DT, Austen KF. Methyltestosterone therapy in hereditary angioedema. Ann Intern Med 1977 March;86(3):306-8. (15) Longhurst HJ, Farkas H, Craig T, ygoren-Pursun E, Bethune C, Bjorkander J et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 2010 July 28;6(1):22. (16) Birjmohun RS, Kees HG, Stroes ES, Hofstra JJ, linga-Thie GM, Meijers JC et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther 2008 December;30(12):2314-23. Short term prophylaxis references • • • • • • (17) Langton D, Weiner J, Fary W. C1-esterase inhibitor concentrate prevents upper airway obstruction in hereditary angio-oedema. Med J Aust 1994 March 21;160(6):383-4. (18) Lehmann A, Lang J, Boldt J, Saggau W. Successful off-pump coronary artery bypass graft surgery in a patient with hereditary angioedema. J Cardiothorac Vasc Anesth 2002 August;16(4):473-6. (19) Leimgruber A, Jaques WA, Spaeth PJ. Hereditary angioedema: uncomplicated maxillofacial surgery using short-term C1 inhibitor replacement therapy. Int Arch Allergy Immunol 1993;101(1):107-12. (20) Mohr M, Pollok-Kopp B, Gotze O, Burchardi H. [The use of a C1-inhibior concentrate for short-term preoperative prophylaxis in two patients with hereditary angioedema]. Anaesthesist 1996 July;45(7):626-30. (21) Pasto CL, Bordas OJ, Mercadal OG, Perez dl, V, Jodar MR. [Profhylaxis and treatment of hereditary and acquired angioedema at HUB; use of the C1-esterase inhibitor]. Farm Hosp 2003 November;27(6):346-52. (22) Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol 2005 March;139(3):379-94. 7.0 Chronic Prophylaxis • Long-term (chronic) prophylaxis refers to the use of regular medication to prevent episodes of angioedema in those with confirmed HAE 7.1 Chronic Prophylaxis: Indication Uniform criteria not established-consider if: • severe events occur more than once/month • patient is disabled for more than 3-5 days per month on average • patient has frequent and severe attacks • Patient can not improve their quality of life enough with on demand therapy 7.1 • Recommendation: The single best recommendation is chronic prophylaxis is indicated when on demand therapy fails to significantly improve the quality of life of a patient with HAE (100%) 7.2 Chronic Prophylaxis: Antifibrinolytics • Recommendation: Anti-fibrinolytics have little benefit, have adverse events and are not indicated for use for prophylaxis of HAE (65%) • Recommendation: The minority recommended use in pre-puberty children for HAE prophylaxis (35%) • Recommendation: Avoid use of anti-fibrinolytics during pregnancy and lactation (80%) • Recommendation: The minority recommended use during pregnancy and lactation (35%) 7.2 Chronic Prophylaxis: Antifibrinolytics • Anti-fibrinolytics may have a place in the management of pre-pubertal children when: - attenuated androgens are relatively contraindicated - C1-INH is not available and - on demand therapy is not effective in controlling the disease. 7.2 Chronic Prophylaxis: Antifibrinolytics, Tranexamic acid and Aminocaproic acid • Mechanism of action is unclear • Tranexamic acid has no effect on C1 INH levels • The dose of Tranexamic acid is 30-50mg/kg daily divided into 2 or 3 doses (this can result in up to 10 large tablets per day) 7.2 Chronic Prophylaxis: Antifibrinolytics Side effects include: • GI upsets • myalgia and CK elevation • theoretical risk of thrombosis 7.2 Chronic Prophylaxis: Antifibrinolytics Contraindications/precautions • presence of thrombophilia or increased thrombotic risk • There is little experience with tranexamic acid in pregnancy • Though not contraindicated during breastfeeding there is little experience with the use of antifibrinolytics during lactation. Anti-fibrinolytic use for chronic prophylaxis Advantages Disadvantages • Inexpensive • Availability • Adverse effects • Few data to support effectiveness • Multiple daily dosing • Unsure of status during pregnancy, lactation and pre-puberty 7.3 Chronic Prophylaxis: Androgens • Recommendation: Androgens are effective to control the symptoms of HAE, but secondary to the potential adverse effects the dose should not exceed Danazol 200 mg a day or an equivalent dose of an alternate androgen and the dose should be reduced to the least effective dose (100%) • Recommendation: Androgens should be avoided during pregnancy, lactation and in most children before puberty (100%) 7.3 Use of androgens in long term prophylaxis • Recommendation: The aim of treatment is to minimize the frequency and severity of angioedema episodes and not necessarily to normalize the biochemical parameters (100%) 7.3 Chronic Prophylaxis: Androgensfunction • Androgens increase hepatic synthesis of the C1 INH protein from the remaining normal C1 gene • Response is dose-related but the dose required to suppress angioedema and/or normalize C4 and/or C1 INH levels varies widely between individuals • Therapy should be titrated by clinical improvement 7.3 Chronic Prophylaxis: Androgens Androgens e.g. danazol are effective: • >90% reduction in episode frequency in >70% of patients • 95% reduction in the frequency of laryngeal episodes 7.3.2 Use of androgens in long term prophylaxis in children • Not recommended for long term prophylaxis in children but its long term use in children has been reported and in some cases the benefits outweigh the risks (minority vote) 7.3.2 Chronic Prophylaxis: Androgens in Children • Complicated by potential adverse effects - risk of androgenization - premature puberty - premature closure of epiphyseal plates with limited growth - liver disorders - behavioral problems • There are few studies of the safety of long term danazol treatment in children. 7.3.2 Chronic Prophylaxis: Androgens in Children • Other attenuated androgens such as stanozolol and oxandrolone have been used for HAE prophylaxis • Stanozolol was reported to be better tolerated than danazol in a retrospective survey but is no longer available • Oxandrolone has a lower virilization potential and may be preferable in children, but this is based only on expert opinion 7.3.3 Chronic Prophylaxis: Androgens Danazol In female patients Danazol: • may inhibit ovulation but cannot be relied upon to prevent pregnancy and patients should be counseled to use nonestrogen contraception • not be taken during pregnancy because of the risk of virilization of the fetus • safety during breastfeeding has not been established and they should be avoided 7.3.4 Chronic Prophylaxis: Androgens Danazol Dosing • Recommended dosage regimens vary • commence with a moderate dose (e.g. 100-200mg daily) • increase or decrease on a monthly basis depending on the frequency of episodes until satisfactory control is reached (Budapest protocol) • maintenance dose required to suppress or substantially reduce angioedema varies between 100mg qod and 800mg daily (best 200 mg or less) • The aim of treatment is to minimize the frequency and severity of angioedema episodes and not necessarily to normalize the biochemical parameters Danazol: Efficacy in Long-Term Prophylaxis Freedom From HAE Attacks vs Placebo 88% 98% 100 Attack-Free Courses Cumulative Freedom From HAE Attacks at Varying Dosages 75 95% 56% 50 25 0 11% >1% Placebo Danazol Frank M. Immunol Allergy Clin N Am. 2006;26:653-668. Gelfand JA, et al. N Engl J Med. 1976;295:1444-1448. 200 300 400 600 Danazol Dosage (mg/d) 7.3.5 Chronic Prophylaxis: Androgens – Potential Side Effects • dose related but highly variable • some patients unable to tolerate even low doses whereas others including females tolerate surprisingly high doses for many years without apparent problems • In a recent survey 79% of patients experienced adverse effects from danazol but only 25% discontinued treatment because of these. 7.3.5 Chronic Prophylaxis: Androgens – Potential Side Effects General • • • • • • headaches nausea fatigue constipation myalgias or muscle cramps weight gain 7.3.5 Long-Term Danazol Therapy Increases Atherogenic Indices in Patients with HAE A. LDL/HDL Ratio P<0.0001 8.0 P=0.0013 P=0.8523 6.0 LDL/HDL, Ratio 4.0 2.0 0.0 Patients Taking Danazol Patients Not Taking Danazol Healthy Controls Szeplaki G et al. J Allergy Clin Immunol. 2005;115:864-869. 7.3.5 Risk of Liver Toxicity Associated With Long-Term Danazol Therapy Danazol-associated hepatocellular adenoma in a 69-year-old female patient on long-term HAE prophylaxis (200 mg/d for 20 years) Bork K et al. Lancet. 1999;353:1006-1067. 7.3.5 Chronic Prophylaxis: Androgens – Potential Side Effects Virilization in females • • • • • • • hirsutism acne voice changes decreased breast size altered libido menstrual irregularities Clitoromegaly • Increase muscle mass 7.3.5 Chronic Prophylaxis: Androgens – Potential Side Effects Hepatic • abnormal liver enzymes, hepatic necrosis, cholestasis, adenoma, carcinoma Metabolic • hypertension, dyslipidemia (but not hypercholesterolemia), atherogenesis, polycythemia, hyperglycemia 7.3.6 Monitoring for adverse events when using androgens for Chronic Prophylaxis (100%) Before use and every 6 months: • Fasting lipid profile • Liver function studies and biochemistry • Complete blood cell count • Urinalysis • Alfa-fetal protein Before use and every 12 months: • abdominal liver ultrasonography 7.4 Use of C1-INH for chronic prophylaxis • Recommendation: C1-INH that is human plasma derived can be used at 1000 units IV twice a week to suppress HAE attacks. Doses as low as 500 units may be effective in some and others may require greater than 1000 units. (100%- rating A) • Recommendation: Human derived C1-INH products should be equally effective, but the shorter half-life of recombinant C1-INH may limit its use for chronic prophylaxis (90%) 7.4 C1-INH for chronic prophylaxis • Recommendation: Dosing may need to be adjusted since even at 1000 units twice a week acute attacks occur in the majority of patients. (90%) • Recommendation: An HAE attack action plan is necessary despite the use of chronic prophylaxis since complete suppression of attacks with chronic C1-INH is unlikely. (100%- A rating) 7.4 C1-INH chronic prophylaxis • Recommendation: during lactation, pregnancy and early childhood if on demand therapy is not effective to control the disease, and chronic prophylaxis is necessary, C1-INH is the preferred therapy. (100%) 7.4.1 Indications for Chronic Prophylaxis with C-1-INH • Angioedema attacks have been frequent and/or dangerous despite on demand therapy or quality of life is still significantly compromised despite on demand therapy And • Prophylaxis with attenuated androgens is unsuccessful, contraindicated or not tolerated 7.4.2 Efficacy of Prophylactic Nanofiltered Plasma C1-INH Concentrate Zuwar NEJM 2009 • Reductions are based on results from a randomized, double-blind, placebo-controlled, multicenter, crossover trial designed to establish the safety and efficacy of routine prophylaxis with CINRYZE™ Normalized Number of HAE Attacks Reduction in Number of Attacks Placebo Zuwar NEJM 2009 CINRYZE™ Dosing C1-INH for chronic prophylaxis • The approved dose is 1000 units twice a week. • At this dose breakthrough attacks are not infrequent • Higher doses and more frequent dosing may be necessary to improve a patients quality of liufe. • Optimal dosing of C1-INH for chronic use has not yet be determined 7.4.2 Chronic Prophylaxis: C-1-INH • The mechanism of action, effects/side effects and monitoring have been discussed under previous sections. Please see section 5.1. • All marketed C1-inhibitor is nano-filtered and can be used interchangeably 7.4.3 Use of C1-INH for chronic prophylaxis • Recommendation: Before starting C1-INH hepatitis B, C, HIV and parvovirus titers should be obtained and monitored on a yearly basis (100%) • Recommendation: Because of the risk of thrombosis with central lines indwelling central lines and catheters should be avoided when administered C1-INH for chronic prophylaxis (100%) 7.4.3 Use of C1-INH for chronic prophylaxis • Because C1-INH is human plasma-derived Hepatitis B and A vaccine should be administered when starting C1INH. (no vote) 7.4.3 Use of C1-INH for chronic prophylaxis Benefits • Effective • Few adverse events • Long safety record from over 3 decades use in the EU Disadvantages • Dose ranging studies are lacking • Human plasma derived • Breakthrough attacks occur despite 1000 units twice a week • Intravenous dosing necessary • Very expensive 7.4.4 Chronic Prophylaxis: Summary 7.4.5 Summary of therapies for chronic prophylaxis Drug Recommendation Efficacy Cost Antifibrinolytics limited indication poor + Androgens Recommended with Very effective precautions Contraindicated in certain populations + C1-inhibitor Recommended +++++ Effective References for chronic prophylaxis • • • • • • • • (1) Degroote DF, Smith GL, Huttula GS. Acute airway obstruction following tooth extraction in hereditary angioedema. J Oral Maxillofac Surg 1985 January;43(1):52-4. (2) Bork K, Barnstedt S-E. Laryngeal edema and death by asphyxiation following tooth extraction in four patients with hereditary angioedema. Am Dent Assoc 2003;134(8):108894. (3) Lodi G, Sardella A, Bez C, Demarosi F, Cicardi M, Carrassi A. Dental experience and self-perceived dental care needs of patients with angioedema. Spec Care Dentist 2001;21(1):27-31. (4) Bork K, Hardt J, Staubach-Renz P, Witzke G. Risk of laryngeal edema and facial swellings after tooth extraction in patients with hereditary angioedema with and without prophylaxis with C1 inhibitor concentrate: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011 July;112(1):58-64. (5) Maeda S, Miyawaki T, Nomura S, Yagi T, Shimada M. Management of oral surgery in patients with hereditary or acquired angioedemas: review and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003 November;96(5):540-3. (6) Maves KK, Weiler JM. Tonsillectomy in a patient with hereditary angioedema after prophylaxis with C1 inhibitor concentrate. Ann Allergy 1994 November;73(5):435-8. (7) Malmstrom HS, Hock JM, Sanford C. Dental management of patients with hereditary angioedema: report of case. J Am Dent Assoc 1985 December;111(6):957-8. (8) Bouillet L, Longhurst H, Boccon-Gibod I, Bork K, Bucher C, Bygum A et al. Disease expression in women with hereditary angioedema. American Journal of Obsterics and Gynaecology 2008;199(5):484. References for chronic prophylaxis • • • • • • • • (9) Farkas H. Pediatric hereditary angioedema due to C1-inhibitor deficiency. Allergy Asthma Clin Immunol 2010 July 28;6(1):18. (10) Farkas H, Gyeney L, Gidofalvy E, Fust G, Varga L. The efficacy of short-term danazol prophylaxis in hereditary angioedema patients undergoing maxillofacial and dental procedures. Journal of Oral and Maxillofacial Surgery 57[4], 404-408. 1999. Ref Type: Abstract (11) Atkinson JC, Frank MM. Dental management of hereditary angioedema. Journal of Oral Pathology and Medicine 20, 139-142. 1991. Ref Type: Abstract (12) Marques L, Domingo D, Maravall FJ, Clotet J. Short-term prophylactic treatment of hereditary angioedema with icatibant. Allergy 2010 January;65(1):137-8. (13) Bowen T, Cicardi M, Farkas H, Bork K, Longhurst HJ, Zuraw B et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol 2010 July 28;6(1):24. (14) Sheffer AL, Fearon DT, Austen KF. Methyltestosterone therapy in hereditary angioedema. Ann Intern Med 1977 March;86(3):306-8. (15) Longhurst HJ, Farkas H, Craig T, ygoren-Pursun E, Bethune C, Bjorkander J et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 2010 July 28;6(1):22. (16) Birjmohun RS, Kees HG, Stroes ES, Hofstra JJ, linga-Thie GM, Meijers JC et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther 2008 December;30(12):2314-23. References for chronic prophylaxis • • • • • • (17) Langton D, Weiner J, Fary W. C1-esterase inhibitor concentrate prevents upper airway obstruction in hereditary angio-oedema. Med J Aust 1994 March 21;160(6):383-4. (18) Lehmann A, Lang J, Boldt J, Saggau W. Successful off-pump coronary artery bypass graft surgery in a patient with hereditary angioedema. J Cardiothorac Vasc Anesth 2002 August;16(4):473-6. (19) Leimgruber A, Jaques WA, Spaeth PJ. Hereditary angioedema: uncomplicated maxillofacial surgery using short-term C1 inhibitor replacement therapy. Int Arch Allergy Immunol 1993;101(1):107-12. (20) Mohr M, Pollok-Kopp B, Gotze O, Burchardi H. [The use of a C1-inhibior concentrate for short-term preoperative prophylaxis in two patients with hereditary angioedema]. Anaesthesist 1996 July;45(7):626-30. (21) Pasto CL, Bordas OJ, Mercadal OG, Perez dl, V, Jodar MR. [Profhylaxis and treatment of hereditary and acquired angioedema at HUB; use of the C1-esterase inhibitor]. Farm Hosp 2003 November;27(6):346-52. (22) Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol 2005 March;139(3):379-94. 8.0 Preventive and long term care of the patient with HAE • Recommendation: All patients with HAE should have an action plan and product available to treat an attack of HAE. (100%) 8.0 Preventive and long term care of the patient with HAE • Recommendation: All patients with HAE should have at least an annual assessment by an HAE specialist. (100%) 8.0 Preventive and long term care of the patient with HAE • Recommendations: During pregnancy and lactation C1INH is considered the preferred therapy. During the third term of pregnancy androgens can be used for short term prophylaxis, but is a second line therapy. (100%) • Androgens, antifibrinolytics, ecallantide and icatibant may be safe during lactation and pregnancy, but use should be discouraged until more safety data are available. (no vote) 8.0 Preventive and long term care of the patient with HAE • All HAE patients have a potential for receiving human blood products. Because of this all HAE patients should be screened as early as possible for Hepatitis B and C and HIV. • In addition, vaccination for Hepatitis B and possibly A should be stressed. • Annual assessment for infections with hepatitis and HIV are suggested. (100%) 8.0 Follow-up appointments • Newly diagnosed patients and those on long term prophylaxis should be seen at 3 month initially and then twice to once a year. • Evaluation at visits should include recording of type and frequency and severity of symptoms, frequency of use and effectiveness of treatments. A comprehensive history, physical examination and appropriate laboratory evaluation should be conducted. • Action plan should be updated and reviewed 8.1 Triggers and trigger avoidance • Triggers of attacks include medication (estrogen contraceptives, estrogen-containing hormone replacement therapy, ACE-Inhibitors), dental work / minor surgery / local anesthesia, stress, infections, menstrual cycle, alcohol. • EVIDENCE LEVELS: C / case series, extrapolation from retrospective studies • Trigger avoidance may reduce number and/or severity of attacks. • EVIDENCE LEVELS: D / expert opinion 8.2 Drugs to avoid in HAE • Estrogen birth control • Estrogen hormone replacement • ACE-inhibitors for blood pressure, CHF and other diseases • Agreement: 100% 8.3 Vaccines • Recommendation: All patients with HAE should receive Hepatitis B vaccine and Hepatitis A vaccine since the chances of receiving blood products is very high • (100% agreement) LEVEL OF EVIDENCE: D / expert opinion • All other vaccines for the general public should be given as indicated • Annual influenza vaccine is indicated since URI can trigger upper airway swelling 8.4 When using C1-INH • Recommendation: Because of the risk of thrombosis with central lines indwelling central lines and catheters should be avoided when administered C1-INH for chronic prophylaxis (100%) 8.5 Monitoring while receiving treatment with Androgens Before Follow up Liver Functional Tests Every 6 months Lipid profile Every 6 months Urine analysis Every 6 months Abdominal ultrasound Once/year Alpha fetoprotein Every 6 months CBC Every 6 months 8.6 Care while on treatment with Plasmin Inhibitors (anti-fibrinolytics) Before Follow up Liver Functional Tests Every 6 months Renal function Every 6 months CPK Every 6 months Eye pressure Annual Tests for thrombophilia Before starting 8.8 summary of screening tests for drug safety Tests Androgens Plasmin inhibitors Liver Functional Tests Q 6 mth Q 6 mth Lipid profile Q 6 mth Renal function Q 6 mth Urine analysis Q 6 mth Abdominal ultrasound Q 12 mth Alpha fetoprotein Q 6 mth Q 6 mth CPK Q 6 mth Eye pressure Q 6 mth Thrombophilia tests? At start of therapy Serology for HIV, hepatitis B, C, E, Parvovirus Plasma derived C1INH/ Plasma Start of therapy and every year 8.7 Family Screening • Screening of all siblings, parents, and children in immediate and extended family with a C4 is recommended. If suspicion is high or C4 screening is abnormal then C1INH protein and function should also be done. If C4 is normal during asymptomatic period, repeating when in an attack may be helpful because of the increase sensitivity. • EVIDENCE LEVELS: D / expert opinion • 100% 8.9 References for preventive therapy • • • • • • • • • Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med 1976;84:580-93. Bowen T, Cicardi M, Farkas H. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema Allergy, Asthma & Clinical Immunology 2010, 6:24. Gargnano Consensus (? will this be available for reference by publication date? Bork K, Staubach P, Eckardt AJ, Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol. 2006;619– 27. Bork K, Ressel N. Sudden upper airway obstruction in patients with hereditary angioedema. Transfus Apheresis Sci. 2003;29:235–238. Bowen T, Cicardi M, Farkas H. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema Allergy, Asthma & Clinical Immunology 2010, 6:24. Waytes AT, Rosen FS, Frank MM: Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med 1996, 334:1630-1634. Kunschak M, Engl W, Maritsch F, Rosen FS, Eder G, Zerlauth G, Schwarz HP: A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion 1998, 38:540-9. 8.9 References for preventive therapy • • • • • • • Craig TJ, Levy RJ, Wasserman RL: Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009, 124:801-8. Zuraw B, Cicardi M, Levy RJ: Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010 Oct;126(4):821-827.e14. Cicardi M, LevyR, McNeil D: Ecallantide for the Treatment of Acute Attacks in Hereditary Angioedema. N Engl J Med 2010 N Engl J Med 2010;363:523-31. Sheffer AL, Campion M, Levy RJ: Ecallantide (DX-88) for acute hereditary angioedema attacks: integrated analysis of 2 double-blind, phase 3 studies. J Allergy Clin Immunol. 2011 Jul;128(1):153-159.e4. Epub 2011 Apr 9. Cicardi M, Banerji A, Bracho F. Icatibant, a New Bradykinin-Receptor Antagonist, in Hereditary Angioedema. N Engl J Med 2010 363;6. Lumry WR, Li H, LevyRJ: Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial, Ann Allergy Clin Immunology, 2011 In Press. Aygoeren-Puersuen E, Martinez-Saguer I, Rusicke E, Kreuz W: On demand treatment and home therapy of hereditary angioedema in Germany - the Frankfurt experience. Allergy Asthma Clin Immunol 2010, 6:21. 8.9 References for preventive therapy • • • • • • • • Bygum A, Andersen KE, Mikkelsen CS: Self-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefits. Eur J Dermatol 2009, 19:147-51. Levi M, Choi G, Picavet C, Hack E: Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol 2006, 117:904-8. Longhurst HJ, Farkas H, Craig T. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 2010, 6:22. Bouillet L, Hereditary angioedema in women. Allergy, Asthma and Clin. Immun. 2010;6(17). Bork K, Meng G, Staubach P, Hardt J: Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006, 119:267-74. Cicardi M, Bergamaschini L, Cugno M, et al. Pathogenetic and clinical aspects of C1 inhibitor deficiency. Immunobiology. 1998;199:366–376., Frank MM: Hereditary angioedema. J Allergy Clin Immunol 2008, 121: S398-401. Craig T, Riedl M, Dykewicz MS, Gower RG, Baker J, Edelman FJ, Hurewitz D, Jacobs J, Kalfus I: When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol 2009, 102:366-372. Szema AM, Paz G, Merriam L: Modern preoperative and intraoperative management of hereditary angioedema. Allergy Asthma Proc 2009, 30:338-42. 8.9 References for preventive therapy • • • • • • • • Wall RT, Frank MM, Hahn M: A review of 25 patients with hereditary angioedema requiring surgery (see comments). Anesthesiology 1989, 71:309-11. Atkinson JC, Frank MM: Oral manifestations and dental management of patients with hereditary angioedema. J Oral Pathology & Medicine 1991, 20:139-142. 26. Farkas H: The efficacy of short-term danazol prophylaxis in hereditary angioedema patients undergoing maxillofacial and dental procedures. J Oral Maxillofac Surg. 1999 Apr;57(4):404-8. Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010a;363:513-522. DeSerres J, Groner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apheresis Sci. 2003;29:247–25. Bowen T, Cicardi M, Bork K: Hereditary angioedema: a current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol 2008, 100(Suppl 2):S3040. Lumry WR, Castaldo AJ, Vernon MK, et al. The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010;31:407-414. 31. Bork K, Bygum A, Hardt J: Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol 2008, 100:153-61. 8.9 References for preventive therapy • • • • • • • • 32. Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR: Screening for liver cancer: results of a randomized controlled trial in Qidong, China. J Med Screen 2003, 10:204-209. 58. 33. Zhang BH, Yang BH, Tang ZY: Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004, 130:417-422. 34. Lunn M, Santos C, Craig TJ. Is there a need for clinical guidelines in the United States for the diagnosis of hereditary angioedema and the screening of family members of affected patients? Ann Allergy Asthma Immunol. 2010 Mar;104(3): 211-14. 35. Zuraw BL. Hereditary Angioedema. NEJM. 2008;359(10):1029. 36. Riedl M, Gower RG, Chrvala CA. Current medical management of hereditary angioedema: results from a large survey of US physicians. Ann Allergy Asthma Immunol. 2011;106:316-322. 9.1 Why Children with HAE are Unique Family History Positive family history (in 75-85% of cases) may assist early diagnosis, before the onset of characteristic symptoms. Complement Testing Complement levels are influenced by age, birth weight, and gestational age. Antigenic and functional C1 inhibitor levels correspond to 70% and 61.8% of adult values initially and increase to the normal level by the age of 6 months to one year. Initial screening should be done after one year. Genetic Testing • • • Not routinely used in clinical practice (uncertain cases, prenatal diagnostics). It is expensive and available in a few health care centers only. No mutation of the C1-INH gene can be detected in 8 to 10 per cent of cases. In children, the possibility of C1 INH deficiency should never be ruled out until the final diagnosis is established. 9.1 Why Children with HAE are Unique Initial Symptoms • usually occur between 5 and 11 years with 50% have symptoms during • • • childhood extremely uncommon during infancy the frequency and severity of symptoms may increase during adolescence especially around puberty the earlier the onset of symptoms, the more likely the HAE will be severe during life Subcutaneous Edema • the most common and the earliest symptom • erythema marginatum is more frequent and is often mistaken for urticaria Upper Airway Edema • Asphyxia may ensue more rapidly in children, because of smaller airway diameter. The earliest occurrence was described in a 3-years-old infant. It rarely is an initial manifestation. 9.1 Why Children with HAE are Unique Gastrointestinal Edema It can mimic an ‘acute abdominal catastrophe’. Estimating the prevalence of GI edema in the pediatric population is difficult, because ‘belly ache’ is a common symptom – especially in infants. Differential Diagnostic Candidates Acute appendicitis, mesenteric lymphadenitis, intestinal invagination, strangulation ileus (intestinal torsion), perforated Meckel’s diverticle, ovarian torsion, etc. Abdominal ultrasound is a sensitive, rapid, and non-invasive differential diagnostic method, especially in children. CT has risk of irradiation, but may be necessary to exclude acute surgical abdomen 9.1 Why Children with HAE are Unique Teachers and health care personnel responsible for the child at school should be informed in writing of the diagnosis. Special medication and an action plan for emergency treatment should be made available at home, at school, and field trips. A proportion of attacks can be prevented through appropriate counseling and lifestyle modifications aimed at eliminating triggering factors. Stigmatization by peers is more frequent in children, than in adults. 9.1 Why Children with HAE are Unique Identification and – when possible – elimination of the following: Triggering Factors • • • • • • The incidence of these factors differs slightly in pediatric and in adult patients. Infection and mechanical trauma are more common during childhood. Compulsory and recommended vaccinations for children are safe and the prevention of infections may reduce the frequency of edematous attacks. Treatment with ACEI is less often necessary during childhood; however, should be avoided. Early initiation of oral contraceptive use is increasingly common. but should be avoided in HAE. The use of estrogens to treat acne is contraindicated in HAE Good dental hygiene is indicated to prevent unnecessary dental procdures 9.1 Treatment of HAE attacks in Children Drugs and the indications for their use in children. • The majority of children do not require long-term prophylaxis (LTP). • Anti-fibrinolytics have minimal benefit • Androgens can cause significant adverse events in children and should be used only in severe cases uncontrolled by other therapies because of adverse events that include: – – – – – – liver damage proatherogenic Masculinization hypogonadism and menstruation irregularities effects on psychic functions and behavior reduction in ultimate body height may occur owing to the premature closure of epiphyseal plates 9.1 Treatment of HAE attacks in Children Drugs and the indications for their use are the same as in adults. Short-Term Prophylaxis (STP) • • • Short term use of androgens is tolerated well. Preferred pre-procedural treatment is with C1-inhibitor. FFP can be utilized, but only in cases C1-inhibitor is not available. Chronic Prophylaxis • In severe cases uncontrolled by on “demand therapy of acute attacks” chronic prophylaxis may be necessary • C1-inhibitor prophylaxis is preferred in children • Androgens can be used, but toxicity often exceeds benefits and should be reserved only in severe uncontrolled cases where other therapies are not available. • Anti-fibrinolytics have minimal efficacy, but may be tried in children 9.1 Treatment of HAE attacks in Children • C1-INH concentrate is effective and safe. • Probably the best dose should be based on 20 units/kg since other weight based dosing is not available • No significant experience is available with the use of ecallantide, icatibant or rcC1-INH in the pediatric population and these drugs should be avoided until more data are available or if benefit outweighs risk. 9.1 Children should be encouraged to have home and self therapy • It is the treatment with the quickest possible action. • It eliminates delays in intervening, as well as the removal of the child from the comforting home environment. • Medication is best administered at home to reduce absenteeism from school • Models from the hemophiliac communities suggest self administration of therapy by children at 8 years and above is effective and safe after appropriate training 9.2 Children with recurrent swelling or abdominal pain • Recommendation: Patients with isolated angioedema, especially if associated with abdominal pain, should be tested to determine if HAE accounts for their symptoms. If confirmed the family should also be tested. (100%) 9.3 Screening for HAE in children • Recommendation: Screening children for HAE should be deferred until after the age of 12 months. (100%) 9.4 Prenatal Diagnosis • Prenatal diagnosis is not recommended because: (75% agreement) – Prediction of severity of disease based on genotyping is not possible. – Many new interventions will allow a much better quality of life – Genetic testing is not absolute – Appropriate reference levels of C4 for fetuses is not known 9.5.1 Treating children • Recommendation: • For children therapy should be based upon weight in HAE • Pharmaceutical companies developing drugs to treat HAE should be encouraged to develop trials for pediatric dosing • At this time, the preferred therapy for children is C1INH. In adolescents androgens can be used, but are second or third line therapeutic options. (100%- expert opinion) 9.5.2 Children need action plans • Recommendation: Similar to adults all children need a treatment action plan, on demand therapy and in rare cases may need prophylaxis. (100%) 9.5.3 Dosing on demand C1-inhibitor • Recommendation: The preferred therapy for ondemand therapy is C1INH dose at 20 units per kg (75%) • Minority opinion suggested 500 unit dosing with repeated dosing as necessary 9.5.4 Chronic Prophylaxis in children • The majority of children do not require long-term prophylaxis • Aminocaproic acid is dosed 20 to 40 mg/kg, but efficacy is questionable • Androgens have significant adverse effects in children. Dose is 2.5 mg/kg of danazol, but dose should not exceed 200 mg a day • Dose for C1-INH should be 20 units/kg twice a week and is the preferred prophylactic therapy 9.5.5 Pediatric references • • • • Gompels MM, Lock RJ, Abinun M et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol 2005; 139(3):379-94. Farkas H, Varga L, Szeplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics 2007; 120(3):e713-22. Farkas H, Jakab L, Temesszentandrasi G et al. Hereditary angioedema: a decade of human C1inhibitor concentrate therapy. J Allergy Clin Immunol 2007; 120(4):941-7. Martinez-Saguer I, Rusicke E, Aygören-Pürsün E, Kreuz W. Clinical surveillance program of pediatric hereditary angioedema (HAE) patients undergoing home treatment AAAAAI 2009. Washington, DC, United States, 2009.) Farkas H. Pediatric hereditary angioedema due to C1-inhibitor deficiency. Allergy Asthma Clin Immunol. 2010 Jul 28;6(1):18.) 10.1 Why Pregnancy is Unique • Anatomical and physiological – primarily hormonal – changes during pregnancy may: • influence the manifestations of HAE, • interfere with the diagnosis of HAE. • Affect the response to therapy. • The range of medicinal products available for safe therapy is narrow, owing to the need for avoiding neonatal undesirable effects. References: - limited - case reports, - retrospective studies involving a small number of patients and pregnancies, reviews, consensus documents 10.1 Why Pregnancy is Unique Pregnancy • It can mitigate, aggravate HAE – or have no impact whatsoever. • Attacks are the most severe during the 1st trimester and occur with the highest frequency in the 3rd trimester; however, decreased attack rates have also been reported during the 2nd and 3rd trimesters. • Attack frequency observed during previous pregnancies does not predict HAE events during any subsequent pregnancy. • Fewer symptoms can occur during the 3rd trimester, if menstruation has been a provoking factor previously. • Early onset of initial symptoms is associated with an increased frequency and severity of attacks. • Abdominal attacks occur more frequently. 10.1 Why Pregnancy is Unique Labor and delivery: • Delivery only rarely induces an attack, which may occur either during labor or within 48 hours of delivery. • The proportion of Caesarean sections is not higher, than in the general population. • C section should be avoided if possible • Intubation should be avoided if possible 10.1 References • • • • Agostoni A, Medicine (Baltimore).1992 Jul;71(4):206-15. Farkas H, Eur J Clin Pharmacol. 2010 Apr;66(4):419-26. Bork K, Ann Allergy Asthma Immunol. 2008 Feb;100(2):153-61. 1: Bouillet L. Allergy Asthma Clin Immunol. 2010 Jul 28;6(1):17. 10.2 Diagnosis During Pregnancy Infrequently, the manifestations of HAE first occur during pregnancy. • Complement testing – Plasma C1INH level decreases during pregnancy as a consequence of the increase of plasma volume. – In patients with eclampsia and pre-eclampsia, the decrease of C1INH levels is greater during the third trimester of pregnancy. – Transitory low C1INH levels return to normal after delivery repeated C1INH testing is necessary after delivery to confirm the diagnosis of HAE. 10.3 Diagnosis Prenatal Diagnosis Routine use of prenatal diagnostics is impractical. No mutation of the C1-INH gene can be detected in 8 to 10 per cent of cases. Mutation itself is not a valid indication for terminating pregnancy, because: – HAE is a manageable disease – its severity cannot be predicted in advance. 10.4 Diagnosis Preimplantation Diagnosis • PGD allows the selection of in vitro fertilized eggs according to mutational status. Only fertilized eggs without disease-causing mutations are used for implantation into the uterus. • PGD: – expensive – requires hormone therapy – pregnancy rate is low 10.5 Spontaneous Abortion and Premature Labor • No increase in incidence of HAE • Pre-procedural prophylaxis should be administered and/or on-demand therapy should be available depending on procedures required. 10.6 Breastfeeding • May be associated with an increased number of edematous attacks, but breast feedings should be encouraged • Medications selected should be safe for the child. C1inhibitor is presently the medication of first choice during lactation 10.7 • Contraception Estrogens should be avoided – as these can worsen the natural course of HAE. Barrier methods, progesterone and intrauterine device should be preferred. • Anti-fibrinolytics should be discontinued several days before attempting to conceive. • Androgens • Should be stopped 2-3 months before attempted conception, owing to the risk of abnormalities in the sexual differentiation of the fetus. • If a patient becomes pregnant while taking androgens, the androgens should be discontinued and the family informed about the risk of fetal abnormalities • Male HAE patients planning a family should avoid androgens, because androgens reduce male fertility (testicular atrophy, impaired spermiogenesis). • C1INH concentrate is the perferred on-demand and prophylaxis therapy during the period of conception 10.8 Treatment during pregnancy • Recommendation: Because of the lack of safety data with icatibant and ecallantide and the toxicity of androgens and antifibrinolytics during pregnancy, C1INH is the preferred drug during pregnancy and lactation. • (100% expert opinion) 10.8.1 Management Prophylaxis Elimination of Triggering Factors The measures for non-pregnant females apply. Drug Prophylaxis Chronic prophylaxis: • • • • Pd C1-INH concentrate is safe and effective during both pregnancy and lactation. Anti-fibrinolytics are not recommended to use during pregnancy. AFs cross the placenta. They are not teratogenic in animals, but are excreted into breast milk. These drugs are not recommended during breastfeeding. Androgens are not recommended, as these drugs cross the placenta. They cause masculinization of the female fetus, placental insufficiency, and fetal growth retardation. No mutagenicity has been shown in animal models. Excretion into breast milk is unknown, but androgens should be avoided during lactation. Heat-treated fresh frozen plasma (FFP): Only limited data exist on the use of FFP during pregnancy. Use only if no C1-inhibitor is available 10.8.2 Management Prophylaxis Short-Term Pd C1-INH is the drug of choice. If this is not available, htFFP or androgens may be administered • • • • Amniocentesis / chorionic villous sampling: pprophylaxis is recommended, preferably with pd C1INH. Surgical artificial abortion: prophylaxis is recommended with pd C1INH. Alternatively, pd C1INH should be administered immediately for signs of an attack. FFP can be used if C1-INH is not available. Delivery and puerperium: It is recommended to manage childbirth in the hospital setting. Routine administration of prophylaxis before uncomplicated natural delivery is not recommended, but pd C1INH should be immediately available. C1-INH concentrate is recommended before labor and delivery when HAE is severe, if symptoms have been recurring frequently during the third trimester, and if the patient’s history contains genital edema caused by frequent mechanical trauma. Administration of pd C1INH concentrate is recommended if forceps delivery or vacuum extraction is performed. After vaginal delivery, patients should be monitored carefully for the initial 48 hours postpartum. Cesarean section (abdominal delivery): Epidural anesthesia and prophylaxis with pd C1INH concentrate are recommended. 10.8.3 On-Demand Therapy during Gestation and Lactation • C1-inhibitor is the preferred therapy. Presently, until further information is available the dose would be 20 units/kg (majority). Minority opinion is 500 or 1000 units for an attack (minority opinion) • FFP (heat treated) can be substituted if C1-inhibitor is not available, but risk is greater than with C1-inhibitor • Anti-fibrinolytics should be avoided • No data are available for ecallantide or icatibant and both should be avoided until safety data exists 10.8 References- Prophylaxis during Pregnancy • • • • Obtulowicz K, Porebski G, Bilo B, Stobiecki M, Obtulowicz A. Hereditary angioedema in pregnancy – case series study. The 6th C1-INH Deficiency Workshop, 22-24 May, Budapest, abstract book page 58. Bouillet L Am J Obstet Gynecol. 2008 Nov;199(5):484.e1-4 Czaller Eu J Obstet Gynecol Reprod Biol 2010, Martinez Am J Obstet Gynecol 2010, 10.10 Cost-Effective Care • Although pd C1-INH is expensive, this is the only agent safe for both the mother and the fetus,and as well its effectiveness is outstanding 10.11 References for Care during Pregnancy • • • • • • • • • • • • • • • • Martinez Am J Obstet Gynecol 2010, Greaves MW. J R Soc Med. 1984 Dec;77(12):1046-8. Farkas H, J Allergy Clin Immunol. 2007 Oct;120(4):941-7. Czaller Eu J Obstet Gynecol Reprod Biol 2010, Obtulowicz K, Porebski G, Bilo B, Stobiecki M, Obtulowicz A. Hereditary angioedema in pregnancy – case series study. The 6th C1-INH Deficiency Workshop, 22-24 May, Budapest, abstract book page 58.) Chinniah N, Aust N Z J Obstet Gynaecol. 2009 Feb;49(1):2-5. 41. Farkas H, J Allergy Clin Immunol. 2007 Oct;120(4):941Bowen T, Ann Allergy Asthma Immunol. 2008 Jan;100(1 Suppl 2):S30-40, McGlinchey PG,. Ulster Med J. 2000 May;69(1):54-7. Logan RA, Greaves MW. Hereditary angio-oedema: treatment with C1 esterase inhibitor concentrate. Acute attack J R Soc Med. 1984 Dec;77(12):1046-8. Czaller Eu J Obstet Gynecol Reprod Biol 2010, Gorman PJ. Can Fam Physician. 2008 Mar;54(3):365-6. Prematta M, Ann Allergy Asthma Immunol. 2007 Apr;98(4):383-8.) Gompels MM,. Clin Exp Immunol. 2005 Mar;139(3):379-94. Carbella, Teresa et al. JACI 2011 11.1 Home Therapy • Recommendation: Patients with HAE should be encouraged to provide self care and home care to allow early, effective and cost effective care • Agreement: 100% • A evidence Self-administration: C1 INH 1000 IU Levi M et al. 20061 1Levi M et al. J Allergy Clin Immunol 2006;117 (4):904-908 Self-administration: C1 INH 1000 IU Levi M et al. 20061 Self-admin Historical control 1Levi M et al. J Allergy Clin Immunol 2006;117 (4):904-908 Self-administration: Results Bygum 20091 Dermatology Life Quality Index (DLQI) scores before and after selfadmin of C1 INH 1Bygum Δ SF36 95%CI pvalue Physical component 21.01 15.29-26.73 0.0001 Psychological component 19.91 9.58-30.23 0.0033 A et al. Eur J Dermatol. 2009 Mar-Apr;19(2):147-51. 11.2 Social and Psychiatry Support • Recommendation: HAE is a physical disease, but the psychiatric toll of the disease is significant and care should be holistic and include the social and psychiatric aspects of the disease. 100% agreement • Patients with HAE suffer from stress and depression more often then matched controls. Stress has been shown to increase severity of disease. Patients may benefit from stress reduction techniques and therapies to improve quality of life. 11.3 Patient Support Organizations • Recommendation: Patients should be encouraged to join groups such as the International Hereditary Angioedema Association • Agreement: 100% 11.4 Database • Recommendation: International database should be developed and all patients encouraged to register so that data are available to help determine the demographics, expression of the disease and variables that affect the expression of the disease. • Agreement: 100% 11.5 Action Plans • Recommendation: All patients with HAE should have an acute action plan to include location to acquire care, therapies available or in possession, dose and route of administration • Agreement: 100% 11.6 Need for Better Medications • Recommendation: Pharmaceutical companies should be encouraged to develop more effective, less toxic and easier to take medications. • Agreement: 100% 11.6 Better medications • Pharmaceutical companies should be encouraged to preform pediatric studies to determine appropriate weight based dosing and side effects of presently available drugs and future medications. • 100% 11.7 World Wide Access to Therapies • Recommendation: Associations, doctors, patients, health care providers, and pharmaceutical companies should petition to have therapies available world wide for all patients with HAE. • Agreement: 100% 12.0 Future Needs for HAE Include • • • • • • Prophylaxis therapy that is oral Dose ranging study for C1INH prophylaxis Subcutaneous C1INH for long term prophylaxis Oral therapy for on demand therapy Pediatric safety studies for icatibant and ecallantide Weight based therapy for all available treatments for pediatric use 12.0 Future Needs for HAE Include • Comparative studies between the treatments for on demand therapy • Comparative studies on prophylactic therapies • Availability of all therapies to all patients around the world • Emphasis on self care • Emphasis on early therapy • Emphasis on action plans for all patients • Gene therapy research 13.0 Summary • We hope this slide set, the abbreviated slide deck and the manuscript that accompanies these are helpful. All are intended for the better dissemination of knowledge about HAE and treatment of patients with HAE. These slides are available on the WAO website to be used for self teaching, patient care and teaching of others. Feel free to use them as you see fit.