Percent Composition
advertisement

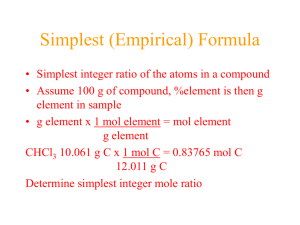
PERCENT COMPOSITION Text 4.5: Page 178-184 Agenda 1. 2. 3. Homework Review Percent Composition: Fertilizer Use Teacher-Led Discussion Percent Composition Calculated by Mass Calculated by Chemical Formula 4. 5. Cookie Chemistry Homework Assignment Learning Goal Students will be able to calculate the percent composition from Masses Chemical formulas Fertilizer on Sale! Percent Composition Is the mass percent of a element in a compound How much one thing is made of another There are 2 ways we can calculate percent composition: Experimentally Theoretically Experimentally EX: 2H + O H2O Determine the mass of the products &reactants MH= 2.5 g MO= MH20= 22.5g Experimentally Determine the Percent Composition % Composition x = (Mx/ Mproduct) x 100 If MH= 20.0 g and MH20= 22.5g Theoretically EX: Na2CO3 Determine the mM of each element mM Na = mM C = mM O = Theoretically EX: Na2CO3 Determine the mM of the compound mM Na = 22.99 g/mol mM C = 12.01 g/mol mM O = 16.00 g/mol mM Na2CO3 = 105.99 g/mol Theroetically Determine the % composition % Composition x = (mMx/ mMproduct) x 100 mM Na = 22.99 g/mol mM C = 12.01 g/mol mM O = 16.00 g/mol mM Na2CO3 = 105.99 g/mol Theoretically: KMnO4 What is the percent composition of K in KMnO4? Homework Assignment Snickerdoodles Homework! EMPIRICAL FORMULAS & MOLECULAR FORMULAS Text 4.5 & 4.7: Page 185-193 Agenda 1. 2. 3. Homework Review CSI: Unknown Identification Teacher Led Discussion 4. Practise Problems 5. Empirical Formula Molecular Formula Evidence Analysis Homework Learning Goals Students should be able to calculate the empirical formula when given the percent composition of a sample Students should be able to calculate the molecular formula when give the percent composition and molecular mass of a sample CSI: Evidence Analysis Empirical Formula A formula that is derived from experimental observations Not theory Tells us the simplest ratio that the elements are combined in Ethyne v. Benzene What is the EF? 1. C6H6 2. C8H18 3. WO2 4. C2H6O2 Finding the Ratio We need to know the percent composition by mass You then convert the mass in grams to moles You can use the moles as the EF’s subscripts This may require multiplication to get whole numbers EX: You test a small sample and find the compound is 60% magnesium and 40% oxygen Practise Problem What is the EF for a compound found to have a percent composition as follows: 21.6% sodium, 33.3% chlorine and 45.1% oxygen. Technology Generally to determine the percent composition Combustion Analysers are used Small sample of substance burned in a combustion chamber When compound burned : O combines with C to make CO2 H combines with O to make H20 vapour All other elements present will convert to oxides Quantities of all these products precisely measures and used to determine % composition But... EF does not necessarily provide correct information about the number of atoms in a molecule Does not tell us the actual quantity of atoms in the sample It tells us the simplest ratio of the atoms Therefore we commonly need the molecular formula Molecular Formula You need to know the mM You then see how many multiples of the EF can fit in the mM Multiply the subscripts by this number to get the MF EX: The EF of a compound is found to be CH3 and the mM is found to be 30.00g/mol . What is the MF? Practise Problem A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole. What is the molecular formula of this compound? Technology Mass spectrometer commonly used Small sample bombarded by beam of e Causes molecules in sample to break up into charged fragments Fragments are accelerated by an electrified field and deflected by a magnetic field Deflection will alter based on mass and charge of fragment Deflection used to determine mM of original sample can be detected Another Practise Problem... Knowing the EF is C3H4O3, what is the MF if the mM is determined to be 176.14 g/n? Homework