LIPID SYNTHESIS
advertisement

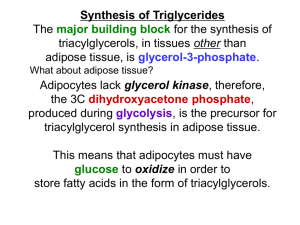
LIPID SYNTHESIS IF THE SHEEP IN THIS PICTURE COULD HEAR TODAY’S LECTURE, THEY MIGHT DECIDE TO RE-THINK THEIR PLANS! HAVING LEARNED ABOUT THE DIFFERENT TYPES OF LIPIDS, THEIR INGESTION, TRANSPORT, STORAGE, SOME PATHOLOGY AND USE AS FUELS – WE NOW TURN TO LIPID BIOSYNTHESIS. THIS IS A VAST AND COMPLEX FIELD, SO FOCUS WILL JUST BE ON FATTY ACID AND CHOLESTEROL SYNTHESES. OTHER AREAS THAT WILL NOT BE APPROACHED ARE SYNTHESES OF SPHINGOLIPIDS, BILE ACIDS AND STEROID HORMONES. FATTY ACID SYNTHESIS – (GENERAL CONSIDERATIONS) IF A LARGE VARIETY OF FATTY ACIDS ARE TAKEN IN, IN THE DIET, AND ARE STORED AND BROKEN DOWN FOR SPECIFIC CELL NEEDS – YOU MIGHT WONDER WHY CELLS GO THROUGH THE PROCESS OF MAKING NEW FATTY ACIDS. IN FACT, IN OUR DIETS THAT ARE COMMON TO THE SO-CALLED “DEVELOPED” NATIONS, THE NEED FOR THE SYNTHESIS OF FATTY ACIDS IS NOT SO CRITICAL, BUT IT DOES OCCUR AND DOES SO IN TWO WAYS: 1) “DE NOVO” SYNTHESIS OF FATTY ACIDS 2) CARBON LENGTHENING OF PALMITIC ACID & FORMATION OF DOUBLE-BONDS “DE NOVO” SYNTHESIS MEANS THE SYNTHESIS OF FATTY ACIDS COMES FROM 2-CARBON UNITS. WHEN IS IT IMPORTANT? UNDER CONDITIONS WHEN THE DIETARY SUPPLY OF FATTY ACIDS IS LIMITED – SUCH AS THE DEVELOPING FETUS; IN COUNTRIES THAT HAVE DECREASED FAT IN THEIR DIET. WHAT “SIDE-EFFECTS” ARE PRODUCED BY DE NOVO SYNTHESIS? SINCE GLUCOSE IS USED TO PRODUCE 2-CARBON UNITS (AS ACETYL CoA), THE 2-CARBON UNITS THAT ARE NOT USED TO MAKE ATP ARE CONVERTED TO FATTY ACIDS BY THE DE NOVO PATHWAY. THIS IS A ONE-WAY PROCESS – MEANING THAT THE CONVERSION OF FATTY ACIDS TO ACETYL CoA CANNOT BE USED TO MAKE GLUCOSE. IN PRACTICAL TERMS, A DIET THAT IS HIGH IN GLUCOSE WILL BE CONVERTED TO FAT IN A SEDENTARY INDIVIDUAL. TISSUE “SITES” OF ACTION FOR DE NOVO SYNTHESIS: LIVER TISSUE (primarily) FAT TISSUE (secondarily) SUB-CELLULAR SITES OF SYNTHESIS: CYTOSOL MITOCHONDRIA CYTOSOL (ASSUMING THAT GLUCOSE IS THE SOURCE) GLUCOSE PYRUVATE ACETYL-CoA CITRATE ACETYL-CoA FATTY ACIDS THESE REACTIONS ARE ENZYMATICALLY CATALYZED THROUGH SEVERAL STEPS. OTHER FATTY ACIDS & AMINO ACIDS MAY ALSO ACT AS SOURCES OF SYNTHESIS. PART I: WHAT IS NEEDED TO BEGIN SYNTHESIS AND WHERE IT COMES FROM THE SYNTHETIC PATHWAY REQUIRES ACETYL CoA AND NADPH. ACETYL CoA MAY ORIGINATE FROM GLUCOSE (BY WAY OF PYRUVATE, SEE THE RED ARROWS), AMINO ACID CATABOLISM (STARVATION CONDITIONS) AND EVEN OTHER FATTY ACID OXIDATION (STARVATION CONDITIONS?). NADPH, A PRODUCT OF THE PENTOSE SHUNT OR FROM MALATE DH ACTIVITY, IS ALSO NEEDED. (The red numbers show what typically occurs.) (6) (4) (5) (7) (2) (3) (8) (1) PART II: FORMATION OF MALONYL-CoA (3CARBON FA FORM) IN ORDER TO BEGIN FATTY ACID SYNTHESIS, SOME ACETYL-CoA MUST BE CONVERTED TO MALONYL-CoA. THIS IS DONE BY CONDENSING BICARBONATE (AS CO2) ON TO ACETYL CoA (SEE ) USING ACETYL CoA CARBOXYLASE. BIOTIN, A CO-ENZYME (AKA VITAMIN B7) IS USED TO TRANSFER THE CO2 FORM TO ACETYL 1 3 CoA. THE ILLUSTRATION SHOWS A DIAGRAM OF 2 THE ACETYL CoA CARBOXYLASE ENZYME THAT HAS 3 POLYPEPTIDE CHAINS. IN THE DIAGRAM, BIOTIN (GREEN ARROW 1) PICKS UP CO2 FROM THE CARBOXYLASE (LEFT, GREEN ARROW 2) & TRANSFERS IT TO ACETYL CoA (GREEN ARROW 3) BY A SWINGING “TETHER” OF THE BIOTIN CARRIER PROTEIN (RIGHT). NOTES ON MALONYL CoA FORMATION: MALONYL CoA FORMATION AND ALL THE FATTY ACID SYNTHESIS STEPS ARE COMMITTED AND POWERED BY THE HYDROLYSIS OF ATP (SEE PREVIOUS SLIDE AT ARROW 2). THE ACTIVITY OF ACETYL-CoA CARBOXYLASE (THE ENZYME THAT MAKES MALONYL CoA) IS THE RATE LIMITING ENZYME OF FATTY ACID SYNTHESIS AND IS NOT A PART OF THE FATTY ACID SYNTHASE MEGAENZYME (TO BE SHOWN). ACTIVATION AND INHIBITION OF ACETYL-CoA CABOXYLASE IS TIGHTLY CONTROLLED AND COMPLEX. THE ENZYME IS AN ALLOSTEIC ENZYME WITH MANY SITES FOR ACTIVATION AND INHIBITION (SEE TEXT pp 726-727). THE HYDROLYSIS OF ATP, PLACES A PHOSPHATE GROUP ONTO A CRITICAL POSITION ON RESIDUE 1200 (LYS) OF THE BIOTIN CARBOXYLASE SUBUNIT (ARROW 2). THE PHOSPHATE BECOMES BOUND TO BICARBONATE, THE TRANSFER FORM OF CO2. INSULIN PROMOTES WHILE EPINEPHRINE AND GLUCAGON INHIBIT THE ENZYME. ALSO THE RELATIVE SUPPLY OF ENERGY (CITRATE = ACETYL CoA) ACTIVATES THE ENZYME WHILE PALMITATE TENDS TO INACTIVATE THE ENZYME, AS DOES EXCESS PHOSPHATE FROM ATP. GENERALLY, THE ACTIVITY OF THE ENZYME IS DETERMINED BY THE SUM OF THE BOUND ACTIVATORS & INHIBITORS AT ANY GIVEN TIME. PART III GENERAL SCHEME OF DE NOVO FATTY ACID SYNTHESIS: THE BIG PICTURE (OPERATION OF THE MEGAENZYME*) PART V: THE FATTY ACID SYNTHASE STRUCTURE (AKA THE “MEGASYNTHASE”) DH= DEHYDRATASE ACP ER= b-ENOYL REDUCTASE KR= b-KETOACYL REDUCTASE TE KS= b-KETOACYL SYNTHASE MAT= MALONYL-CoAACETYL CoA-ACP TRANSACYLASE TE= THIOESTERASE ACP= ACYL CARRIER PROTEIN ACP ACP ACP TE TE TE Reaction chamber Reaction chamber NOTES: ON THE MAMMALIAN ENZYME, THE COMPLETE STRUCTURE IS FORMED AS TWO POLYPEPTIDE CHAINS (ONLY ONE IS SHOWN HERE). THE LOCATIONS OFTHE ACP AND THE THIOESTERASE ARE NOT KNOWN WITH CERTAINTY, BUT THE ACP ISTHOUGHT TO HAVE FLEXIBLE LOCATIONS IN ORDER TO “DELIVER” THE SUBSTRATE TO VARIOUS CATALYTIC SITES (*). THE LABELS CALLED “REACTION CHAMBERS” REALLY INCLUDE ALL THE ACTIVE SITES. PICTURE THE GROWING FATTY ACYL GROUPS BEING MOVED FROM REACTIVE SITE TO REACTIVE SITE BY THE TETHERED ACYL CARRIER PROTEINS. PART IV: PRIMING THE PUMP –THE ACYL CARRIER PROTEIN DOMAIN & TRANSFER OF ACETYL & MALONYL GROUPS. = -C-CH3 O -C-CH3 = NOTE: THE ACYL CARRIER PROTEIN + THE PHOSPHOPANTETHEINE GROUP WILL BE NOTED IN SUBSEQUENT SLIDES AS “ACP” AS IN ACETYL-ACP OR MALONYL-ACP MALONYL-CoAACETYL-CoA—ACP TRANSACYLASE (MAT) O Acetyl CoA PART V: THE 1st CYCLE OF SYNTHESIS MAT Malonyl CoA KS KR ACP MAT= malonylCoA-acetylCoA-ACP transacylase KS=b-ketoacyl synthase KR =b-ketoacyl reductase DH=dehydratase ER=b-enoyl reductase ACP DH ER Recycle reactions PART VI: SUBSEQUENT CYCLES AND RELEASE FIRST CYCLE TO HERE* SECOND CYCLE TO HERE, ETC. *SEE PREVIOUS SLIDE FOR DETAILED REACTIONS. THERE ARE A TOTAL OF 7 CYCLES EACH ADDING 2 MORE CARBONS. SINCE THE SYNTHESIS BEGINS WITH 2 CARBONS, THE TOTAL PRODUCED IS 16 CARBONS AS PALMITATE. PALMITATE IS RELEASED FROM THE ACP IN THE ENZYME BY THE CATALYTIC ACTIVITY OF THIOESTERASE (TE) LOCATED ON THE FATTY ACID SYNTHASE. THIS OCCURS SINCE THE MEGASYNTHASE CANNOT ACCOMMODATE ANY MORE THAN 16 CARBON FATTY ACIDS. TIME OUT! DID YOU EVER WONDER WHAT HAPPENED TO THE WOMAN WHO KISSED THE FROG PRINCE? SHE’S TALKING TO HER FRIEND ON THE PHONE – “OH, YES MARTHA, NOW YOU SHOULD SEE ALL THE TADPOLES I’M STUCK WITH.” A HELPFUL COMPARISON BETWEEN FATTY ACID BASIC METABOLIC MECHANISMS: FATTY ACID b-OXIDATION or (DEGRADATION…last lecture) vs. FATTY ACID CONDENSATION or (SYNTHESIS…this lecture) IN EACH PATHWAY, THE TERM “ACTIVATION” MEANS CARRIER BINDING TO ALLOW ENZYMATIC REACTIONS TO TAKE PLACE: ACETYL CoA FOR DEGRADATION; ACYL CARRIER PROTEIN (ACP) FOR SYNTHESIS. b co2 FATTY ACID SYNTHESIS – (CONTINUATION OF GENERAL CONSIDERATIONS) RECALL THAT IT WAS SAID THAT DIETS COMMON TO THE SO-CALLED “DEVELOPED” NATIONS DO NOT HAVE A CRITICAL NEED FOR THE SYNTHESIS OF FATTY ACIDS, BUT THE SYNTHESIS STILL OCCURS IN TWO WAYS: 1) “DE NOVO” SYNTHESIS OF FATTY ACIDS (JUST DISCUSSED) 2) CARBON LENGTHENING OF EXISTING PALMITIC ACID & FORMATION OF DOUBLE-BONDS NOW LETS LOOK AT THE SECOND PART OF THAT SYNTHESIS: FURTHER CHAIN LENGTHENING AND THE FORMATION OF DOUBLEBONDS. CHAIN LENGTHENING AND DOUBLE-BOND FORMATION (AND ITS LIMITATIONS): PALMITATE LENGTHENING OCCURS IN THE MITOCHONDRIA AND SMOOTH ENDOPLASMIC RETICULUM BY SEPARATE, BUT SIMILAR MECHANISMS. THE LENGTHENING IS USUALLY LIMITED TO 2 ADDITIONAL CARBONS. ALSO: ONLY SINGLE UNSATURATED FATTY ACIDS CAN BE FORMED FROM THE SATURATED FATTY ACIDS. SO, THE PRODUCTS SYNTHESIZED ARE ONLY: PALMITOLEATE (16:1D9) STEARATE (18:0) OLEATE (18:1D9) palmitoleic acid steric acid oleic acid IN FACT, THESE THREE FATTY ACIDS COMMONLY OCCUR IN THEIR ESTER FORMS IN ADIPOCYTES. THE ENZYMES THAT CARRY OUT THESE ACTIVITIES ARE CALLED FA ELONGASES AND FA DESATURASES. GETTING LONGER CHAIN, POLYUNSATURATED FATTY ACIDS REQUIRES ESSENTIAL FATTY ACIDS FROM THE DIET. THIS IS THE POLYUNSATURATED FATTY ACID DISCONNECT FOR HIGHER LIFE FORMS. IN ORDER TO GET LONGER CHAIN, POLYUNSATURATED FATTY ACIDS (PUFAs) THEN, THERE IS A NEED FOR LINOLEATE (18:2D9,12) AND a-LINOLENATE (18:3D9,12,15) AS STARTING FAs. THESE FAs ARE ESSENTIAL FOR HIGHER ANIMALS SINCE THEY CANNOT MAKE (SYNTHESIZE) THESE FATTY ACIDS FROM PALMITATE. LINOLEATE AND a-LINOLENATE MUST BE USED TO MAKE POLYUNSATURATED FATTY ACIDS (e.g. ARACHIDONATE (20:4D5,8,11,14). NOTE: LINOLEATE & ARACHIDONATE ARE AKA w6 FATTY ACIDS WHILE a-LINOLENATE IS AKA w3 FATTY ACID (counting from the methyl end of the fatty acid – a practice used by nutritional biochemists). WHAT ARE SOME OTHER PUFAs AND WHAT ARE SOME OF THEIR PRODUCTS? (EICOSANOID SYNTHESIS) LINOLEATE me ARACHIDONATE me PROSTAGLANDINS me me THROMBOXANES me LEUCOTRIENES AN ABBREVIATED FORM OF EICOSANOID SYNTHESIS FROM ARACHIDONATE. ARACHIDONATE IS RELEASED FROM PHOSPHOLIPIDS ON PLASMA MEMBRANES BY PHOSPHOLIPASE A2. MULTIPLE ENZYMES (me) CONVERT ARACHIDONATE TO PROSTAGLANDINS (PG), THROMBOXANES (TX) OR LEUCOTRIENES (LT). EACH EICOSANOID SERVES AS A SHORT ACTING, LOCAL HORMONE. E.g., PGD2 HAS ANTI-INFLAMMATORY ACTIVITY WHILE LEUKOTRIENE C SUSTAINS INFLAMMATORY REACTIONS. STEROIDS AND “NSAIDS” INHIBIT EICOSANOID SYNTHESIS. NOTES ON THE PATHWAYS TO EICOSANOID SYNTHESIS: 1) LINOLEATE IS CONVERTED TO ARACHIDONATE BY ENZYMES STORED IN THE ENDOPLASMIC RETICULUM BEFORE INCORPORATION INTO PHOSPHOLIPID PLASMA MEMBRANES. 2) PHOSPHOLIPASE A2 BREAKS ARACHIDONATE AWAY FROM PM PHOSPHOLIPIDS. THE ENZYME IS ACTIVATED BY EITHER A HORMONE OR BY MECHANICAL CONTACT & IS INHIBITED BY CORTICOSTEROIDS. 3) PROSTAGLANDIN SYNTHASE IS A COMPLEX OF TWO ENZYMES: AN OXIDASE AND A CYCLASE. IT IS AKA CYCLOOXYGENASE (COX). COX INHIBITORS ARE CALLED NON-STEROIDAL, ANTI-INFLAMMATORY DRUGS (NSAIDS). THE ENZYME IS BOUND TO THE CELL PLASMA MEMBRANE. THE ILLUSTRATION ON THE RIGHT SHOWS AA 33-583 OF COX-1. A BLUE MOLECULE OF IBUPROFEN IS BOUND AT THE ACTIVE SITE OF THE ENZYME AT WHAT IS KNOWN AS THE TUNNEL PORTION OF THE ENZYME. THIS ACTION PREVENTS THE ARACHIDONATE FROM COMPLETING ITS CONVERSION TO PROSTAGLANDIN H2. ANOTHER PUFA WORTHY OF OUR ATTENTION: AN w-3 FATTY ACID THAT HAS GAINED RECENT ATTENTION IS DHA (DOCOSAHEXANENOIC ACID aka CERVONIC ACID). THIS FATTY ACID IS SYNTHESIZED FROM a-LENOLENATE IN THE ENDOPLASMIC RETICULUM AND HAS THE STRUCTURE 22:6D4,7,10,13,16,19). THE HIGH DEGREE OF UNSATURATION (6 DOUBLE BONDS!) MAKES THIS FATTY ACID A DESIRABLE COMPONENT IN THOSE MEMBRANES THAT REQUIRE CONSIDERABLE FLEXIBILITY AS BIOLOGICAL LIQUID CRYSTALS. THIS FLEXIBILITY ALLOWS FOR THE COMPLEX CONDUCTION PROCESSES REQUIRED IN NERVOUS TISSUES. THAT IS, PROTEINS IN THESE MEMBRANES HAVE GREATER FREEDOM TO MOVE WITHIN THE MEMBRANE. APPOXIMATELY 22% OF ALL THE FATTY ACIDS FOUND IN THE RETINA IS CERVONIC ACID. AT THE RIGHT ARE SHOWN 2 POSSIBLE CONFORMATIONS OF CERVONIC ACID IN A MEMBRANE (see arrow). THEREFORE - POINTS TO NOTE ABOUT FATTY ACID SYNTHESIS: FATTY ACID SYNTHESIS (DE NOVO) AND FATTY ACID ELONGATION, ALONG WITH UNSATURATION, DO NOT SEEM TO SERVE HUMANS WITH THE BEST EFFICIENCY SINCE: 1) CARBOHYDRATES ARE SYNTHESIZED TO EXCESS FATTY ACIDS WHEN THEY ARE NOT USED TO MAKE ATP. 2) THE PROCESSES OF ELONGATION & UNSATURATION OF C-16 FATTY ACIDS IS LIMITED SINCE CONVERSION TO PUFAs CANNOT OCCUR. ESSENTIAL FATTY ACIDS, WHICH WE CANNOT MAKE, ARE NEEDED AS STARTING POINTS FOR THE SYNTHESIS OF A VARIETY OF USEFUL, POLYUNSATURATED FATTY ACIDS (AKA PUFAs). SOME EXAMPLES OF PUFAs ARE PROSTAGLANDINS (SHORT ACTING HORMONES) AND DHA (A FATTY ACID THAT MAY BE CARDIOPROTECTIVE, ANTI-INFLAMMATORY AS WELL AS A COMPONENT IN NERVOUS TISSUE FUNCTIONS). A PUFA IS ANY FATTY ACID WITH TWO OR MORE DOUBLE BONDS. WITH VIRTUALLY NO EXCEPTONS IN ANIMAL TISSUE, A PUFA HAS A MINIMAL CARBON LENGTH OF 18. DHA = DOCOSAHEAENOIC ACID. THE SYNTHESIS OF CHOLESTEROL CHOLESTEROL HAS GOTTEN A BAD REPUTATION IN MORE RECENT YEARS DUE TO ITS ASSOCIATION WITH BLOOD VESSEL BLOCKAGE. IN FACT, THIS MOLECULE IS QUITE IMPORTANT AS A COMPONENT OF PLASMA MEMBRANE STRUCTURE; AS WELL AS A PRECURSOR NECESSARY FOR LIPID DIGESTION (BILE SALTS), LIPID SOLUBLE VITAMINS; AND STEROID HORMONES. WE COULD NOT LIVE WITHOUT THIS MOLECULE. THE FIGURES ON THIS SLIDE SHOW YOU DIFFERENT WAYS OF REPRESENTING THIS CHOLESTEROL. ON THE LEFT IS THE FAMILIAR 2-DIMENSIONAL, STRUCTURAL FORMULA. IN THE MIDDLE, IS A DIAGRAM OF ITS THREE MAIN COMPONENTS, AND ON THE RIGHT A van der WAALS REPRESENTATION OF HOW THE MOLECULE MIGHT LOOK IN 3-DIMENSIONAL SPACE. IF YOU WERE TO TURN THE MOLECULE AROUND ON ITS Z-AXIS (VERTICAL AXIS) YOU WOULD SEE THAT IT IS BOTH FLAT AND SOMEWHAT BULKY. WE OBTAIN CHOLESTEROL FROM 2 SOURCES: DIET AND SYNTHESIS. DIETARY CHOLESTEROL INTAKE IS AN AMOUNT THAT CAN BE CONTROLLED (AND IN SOME CASES MUST BE CONTROLLED *) TO AVOID HIGH LDL/HDL LEVELS & POSSIBLE BLOOD VESSEL BLOCKAGE. CHOLESTEROL SYNTHESIS IS A PROCESS OVER WHICH AN INDIVIDUAL HAS NO (?) CONTROL. IN THIS PART OF THE LECTURE, WE WILL CONSIDER HOW THIS PROCESS OCCURS AND WHAT CONTROL MECHANISMS ARE ASSOCIATED WITH CHOLESTEROL SYNTHESIS. *IN CASES OF OVERWEIGHT AND HYPERCHOLESTEROLEMIA WHICH WAS PREVIOUSLY DESCRIBED. SITE & ORIGINS OF SYNTHESIS WITH AN OVERALL DIAGRAM. CHOLESTEROL IS MADE INITIALLY IN THE LIVER CELLS’ CYTOSOL AND THEN IN ITS ENDOPLASMIC RETICULUM. THE 1ST MOLECULES ARE ACETYL CoA, JUST AS WITH FATTY ACID SYNTHESIS. THERE THE SIMILARITY ENDS. EACH STAGE IN THE DIAGRAM TO THE RIGHT REPRESENTS SOME MAJOR INTERMEDIATES: MEVALONATE, ACTIVATED ISOPRENE, AND SQUALENE. THE CIRCLED NUMBERS ARE FOR MULTIPLE-STEP, ENZYME CATALYZED REACTIONS. COMMITMENT BUILDING BLOCK FORMATION CONDENSATION CYCLIZATION THE STEPS IN CHOLESTEROL BIOSYNTHESIS ARE LONG AND COMPLEX. WHY SHOULD THEY BE STUDIED? AS WITH MANY THINGS IN LIFE, IT’S ALL ABOUT CONTROL AND FUNDING TO RESEARCH THE MECHANISM OF SYNTHESIS WAS “SOLD” ON THE BASIS OF EVENTUALLY FINDING WAYS TO PREVENT THE EXCESSIVE SYNTHESIS OF CHOLESTEROL IN THE BODY -- TO PREVENT, FOR EXAMPLE, HEART ATTACKS. HISTORICALLY, THE INVESTIGATIONS TO DETERMINE HOW CHOLESTEROL IS MADE HAVE EXTENDED FROM THE 1940’S EVEN UP TO THE PRESENT DAY SINCE SOME MINOR DETAILS OF ENZYMATIC REACTIONS HAVE YET TO BE DESCRIBED. HERE WE ARE GOING TO TAKE A CAREFUL AND ABBREVIATED LOOK AT THOSE PARTS OF THE SYNTHETIC PATHWAY THAT HAVE YIELDED USEFUL INFORMATION. The commitment stage 1 causes mevalonate to be synthesized from 3 acetyl CoAs by 3 enzymes: thiolase, OH-methyl glutaryl-CoA synthase and OHmethyl glutaryl reductase: Rx 1 2 acetyl CoA thiolase acetoacetyl CoA COMMITMENT BUILDING BLOCK FORMATION synthase Rx 2 acetyl CoA + acetoacetyl CoA 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) CONDENSATION reductase Rx3 HMG-CoA + 2 NADPH + 2 3-mevalonate H+ USEFUL INFORMATION: THE 3RD ENZYME: OH-METHYL GLUTARYL REDUCTASE IS THE RATE LIMITING ENZYME FOR THE ENTIRE PATHWAY OF CHOLESTEROL SYNTHESIS. THE ENZYME IS CONTROLLED BY KINASES/ PHOSPHATASES; CHOLESTEROL LEVELS ON ½ LIFE OF ENZYME AND mRNA LEVELS OF THE ENZYME. CYCLIZATION INHIBITORS FOR HMG-CoA REDUCTASE – A CLINICAL PAYOFF FOR CHOLESTEROL SYNTHESIS STUDIES 3-OH-3-METHYL GLUTARYL-CoA = -E OH-METHYL GLUTARYL-CoA SUBSTRATE STATINS MEVALONATE PRODUCT EXAMPLE OF AN INHIBITOR: LIPITOR, HAVING A STRUCTURE SIMILAR TO THE OH-METHYL-GLUTARYL INTERMEDIATE, BINDS TO THE ACTIVE SITE OF THE REDUCTASE AND INHIBITS ITS CATALYTIC ACTION OH-METHYL-GLUTARYL INTERMEDIATE Building block and condensation stages 2 and 3 proceed through four enzymatic reactions that require ATP to drive the reactions initially to the formation of activated isoprene (seen on the right) AKA isopentenyl pyrophosphate. Squalene formation formation then takes place by enzymatic condensation of six molecules of Isopentenyl pyrophosphate such that: C5 C10 C15 COMMITMENT BUILDING BLOCK FORMATION C30 is formed (5x6=30) The cyclization stage 4 is a multi-step, enzymatic process that adds an OH group to ring A, closes the four rings, adds a double bond to ring B, preserves the methyl group between rings A and B, and forms a new methyl group between rings C and D (short blue arrows). Enzymes in stages 2-4 are not concerned with the rates of cholesterol synthesis. However, regulation of the rate of LDL receptor synthesis & the esterification of cholesterol are also important. CONDENSATION CYCLIZATION 20 Rx’s! C A B D THE LDL RECEPTOR, ITS DEFECTS & BLOOD CHOLESTEROL – A REVIEW WHEN CHOLESTEROL HAS BEEN SYNTHESIZED IN THE LIVER AND ESTERIFIED FOR INCORPORATION INTO LDLs, RECALL THAT IT MUST BE REMOVED FOR CELLULAR DELIVERY BY AN LDL RECEPTOR. THIS REMOVAL IS ONE WAY IN WHICH BLOOD CHOLESTEROL IS CONTROLLED (LOWERED). THE DISEASE CALLLED FAMILIAL HYPERCHOLESTEROLEMIA (mentioned in the Last lecture) INVOLVES AN ABSENCE OR DEFECT IN THE LDL RECEPTOR MOLECULE (several possibilities can occur): a homozygous defect when no receptor is made [>600 mg/dL of blood cholesterol] while a heterozygous defect results in ~1/2 of the receptor being made [>300 mg/dL of blood cholesterol]. IN ADDITION, SEQUENCE MISTAKES IN THE LDL BINDING DOMAIN FAIL TO CAUSE THE RELEASE OF CHOLESTEROL ( ) OR SEQUENCE ANOMALIES IN THE C-TERMINAL DOMAIN ( ) CAUSE A FAILURE IN PIT FORMATION TO INVAGINATE [TAKE UP] THE CHOLESTEROL. IN ADDITION, IT IS ALSO POSSIBLE THAT THE RECEPTOR PROTEIN DELIVERY MECHANISM FROM THE GOLGI APPARATUS TO THE CELL PLASMA MEMBRANE MAY BE DEFICIENT (see green arrow). THIS IS HOW DRUGS LIKE LIPITOR COMBAT THESE PROBLEMS. SUMMARY WE HAVE JUST LOOKED AT 2 PROCESSES ABOUT LIPID METABOLISM: THE SYNTHESIS OF FATTY ACIDS AND THE SYNTHESIS OF CHOLESTEROL. IN ADDITION, THE DEFICIENCIES AND POSSIBLE PATHOLOGIES ASSOCIATED WITH THESE PATHWAYS HAVE BEEN POINTED OUT. WHAT IS IMPORTANT? The hazards of space travel! FATTY ACID SYNTHESIS – 1) THE CHARACTERISTICS OF “DE NOVO” SYNTHESIS ** ITS IMPORTANCE ** ITS “SIDE” EFFECTS (EXCESS FAT FROM GLUCOSE) ** WHERE IT OCCURS ** WHAT IS NEEDED FOR SYNTHESIS ** MALONYL CoA FORMATION & GENERAL SCHEME ** THE MEGASYNTHASE MOLECULE ** THE ACYL CARRIER PROTEIN ** THE 1st CYCLE OF SYNTHESIS (the devil is in the details) ** SUBSEQUENT CYCLES (where does it stop) CAN YOU COMPARE FATTY ACID SYNTHESIS WITH BETA-OXIDATION? 2) THE CHARACTERISTICS OF CARBON LENGTHENING & DESATURATION ** LIMITATIONS OF THIS PROCESS ** THE NEED FOR PUFAs – ESSENTIAL FATTY ACIDS IN THE DIET ** SYNTHESIS OF PROSTAGLANDINS, THROMBOXANES & LEUCOTRIENES -- WHAT ARE THEY & HOW ARE THEY MADE (the devil is in the details)? ** THE IMPORTANCE OF PLA2 & PROSTAGLANDIN SYNTHASE INHIBITORS ** SYNTHESIS OF CERVONIC ACID AND ITS FUNCTION (yes, the devil is here too). THE SYNTHESIS OF CHOLESTEROL – 1) THE IMPORTANCE OF CHOLESTEROL ** IT CONTRIBUTES TO -----** IT IS A SOMEWHAT FLAT MOLECULE WITH THREE PARTS. WHY IS THAT IMPORTANT? 2) THE “EVILS” OF CHOLESTEROL ** HYPERCHOLESTEROLEMIA 3) HOW THE CELLULAR SYNTHETIC PROCESS WORKS ** ACETYL CoA ** IMPORTANT INTERMEDIATES: MEVALONATE, ISOPRENE, SQUALENE ** COMMITMENT STAGE AND CONTROL OF SYNTHESIS. ** INHIBITORS OF HMG-CoA REDUCTASE & THEIR IMPORTANCE -- LIPITOR AS AN EXAMPLE. WHAT DOES IT DO? ** BUILDING BLOCK, CONDENSATION & CYCLIZATION STAGES 4) THE ROLE OF THE LDL RECEPTOR AND CONTROL OF BLOOD CHOLESTEROL ** THE RECEPTOR’S NORMAL ROLE ** BACK TO HYPERCHOLESTEROLEMIA – VARIATIONS IN DEFECTS OF THE LDL RECEPTOR.