Methods of Teaching Predicting Reaction
advertisement

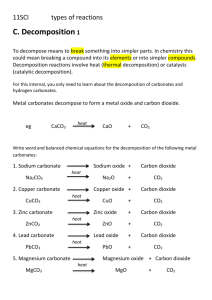
Predicting Reactions Presented by Mr. Mark Langella STANYS 2006 PWISTA.com 11/7/06 Sch ool Di strict h as no teachin g cente r Tea chi ng Cen ter - no t en ough tea che rs to run prog ram Sch ool Di strict -nee ds to su pply at leas t 15 hou rs of teacher train ing Tea chi ng Cente r -Needs prog ram tha t science tea che rs are loo kin g to attend PWISTA -Inn ovative Progra ms for tea che rs Compa ny -loo kin g to dis tribu te equ ipme nt Compa ny -loo kin g to hel p lo cal co mmun ity school s Why do the reactions occur? Gibbs Free Energy drives the Spontaneous reactions Lower PE energy Formation of Stronger Bonds Greater Entropy ( Formation of Gases) Solubility Formation Constant Lose Yourself in Chemical Reactions Google Video Synthesis or Combination Reactions In synthesis or combination reactions, two or more substances combine together to form a single product. The general form is A + B C The products must contain only those elements found in the reactants. Metal + Nonmetal Salt Magnesium ribbon is burned in oxygen Combination or Synthesis Reactions General form A+B C In this demonstration 2 Mg + 3 Mg + N2 O2 MgO Mg3N2 The oxygen and nitrogen occur naturally in the atmosphere O2 is 21% of air N2 is 78% of air Energy Two types of energy are produced in this demonstration energy DHf MgO = -601.83 kJ/mole (kiloJoules per mole) heat this is said to be the ‘heat of formation’ for MgO the negative sign indicates the formation is exothermic light energy approximately 10% of the energy of combustion occurs as light in this demonstration more light than any other known reaction Online Demos Reaction of Iron and Sulfur http://www.pc.chemie.uni-siegen.de/pci/versuche/pics/anim/fes.mpg Fe + S Reaction of Potassium and Oxygen http://neon.chem.ox.ac.uk/vrchemistry/FilmStudio/alkalimetals/HTML/pag e08.htm Reaction of Lithium and Oxygen http://neon.chem.ox.ac.uk/vrchemistry/FilmStudio/alkalimetals/HTML/pag e02.htm Reaction of Lithium and Chlorine http://neon.chem.ox.ac.uk/vrchemistry/FilmStudio/alkalimetals/HTML/pag e04.htm Reaction of Sodium and Oxygen http://neon.chem.ox.ac.uk/vrchemistry/FilmStudio/alkalimetals/HTML/pag e05.htm Reaction of Zinc and Sulfur http://boyles.sdsmt.edu/znsulf/zincsul.htm FeS Nonmetal + Nonmetal compounds Reaction of Hydrogen and Oxygen http://www.chem.uiuc. edu/clcwebsite/video/ Bal2.mov Molecular Rubber tubing Water bottle clamped onto a rinstand Drying tube Short piece of Pyrex glass tubing inserted into a one-hole stopper Volumetric flask containing NaOH and Al Reaction of Phosphorus and Chlorine http://boyles.sdsmt.edu/pwithcl/DirksTwo.as x P4(s) + Oxidation Number Changes 10 Cl2(g) 4 PCl5(s) Nonmetal Oxide + Water Oxyacid Oxy Acid= Contains H+ ions attached to common Polyatomic ion of Nonmetal Oxide plus one more oxygen Formation of Carbonic Acid Carbon dioxide and Water- Carbon Dioxide is easily produced by the reaction of sodium bicarbonate and vinegar. http://boyles.sdsmt.edu/respira/Alexander Co2Blue.asx Reaction of Carbon Dioxide and Limewater http://boyles.sdsmt.edu/respira/Alexander Co2White.asx CO2(g) + Ca(OH)2(l) CaCO3(s) + H2O(l) Metal oxide + water Egg Fry metal hydroxide DECOMPOSITION REACTIONS Substances break down by means of decomposition reactions The general form of a decomposition reaction is C A+B Decomposition reactions are the opposite of combination or synthesis reactions Decomposition of Metal Carbonate Heating a metal carbonate always yields the metal oxide and carbon dioxide. MCO3 MO + CO2 Heating the carbonates Most carbonates tend to decompose on heating to give the metal oxide and carbon dioxde. For example, a typical Group 2 carbonate like calcium carbonate decomposes like this: In Group 1, lithium carbonate behaves in the same way - producing lithium oxide and carbon dioxide. The rest of the Group 1 carbonates don't decompose at Bunsen temperatures, although at higher temperatures they will. The decomposition temperatures again increase as you go down the Group. Metal Hydrogen Carbonate Decomposition Heating a metal bicarbonate gives the metal oxide, carbon dioxide, and water. MHCO3 MO + H2O + CO2 Solid Sodium Hydrogen Carbonate is strongly heated Metal Chlorate Decomposition Heating a metal chlorate gives the metal chloride plus oxygen. MClO3 MCl + O2 Burning Gummi Bears http://www.webct.com/service/ViewContent?cont entID=1249557&communityID=858&categoryID =1249537&sIndex=0 Logger Pro Analysis Decomposition of Ammonium Dichromate http://boyles.sdsmt.edu/dichrom/Ammoniu mDichromate.asx (NH4)2Cr2O7(s) Cr2O3(s) + N2(g) + 4H2O(g) Peroxide Decomposition Elephant’s Toothpaste Website: http://boyles.sdsmt.edu/tpaste/Cain.asx Genie in a Bottle Demo Website: http://boyles.sdsmt.edu/geniebot/genie.htm Reactions Based on Reduction Potentials EMF Potential Reduction and Oxidation Single replacement Cation Replacement There are two types of single replacement reactions, in one, a metal or hydrogen replaces a positive ion M0 + A+B- M+B- + A0 Reaction of Sodium and Water http://boyles.sdsmt.edu/sodwat/SodiumWater.asx http://www.theodoregray.com/PeriodicTable/Stories /011.2/Videos/SodiumResearch03.html http://www.theodoregray.com/PeriodicTable/Stories /011.2/Videos/SodiumResearch02.html Sodium(s) + Water(l) Sodium Hydroxide(aq) + Hydrogen(g) 2Na(s) + 2H2O(l) 2NaOH(aq) + H2(g) Reaction of Potassium and Water http://www.chem.shef.ac.uk/webelementsmoov/K_H2O.mov http://www.theodoregray.com/PeriodicTable/AlkaliBangs/019_K_dog howls.html Potassium(s) + Water(l) Potassium Hydroxide(aq) + Hydrogen(g) 2K(s) + 2H2O 2KOH + H2(g) Group I with water video http://video.google.com/videoplay?docid=2134266654801392897&q=rubidium+water Reaction of Zinc and Tin (II) Chloride http://www.chemtopics.com/lectures/unit02/lectur e1/displace.htm Zinc(s) + Tin (II) Chloride(aq) Tin(s) + Zinc (II) Chloride(aq) Zn(s) + SnCl2(aq) Sn(s) + ZnCl2(aq) Zinc(s) + Hydrochloric Acid(aq) Zinc (II) Chloride(aq) + H2(g) Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) Aluminum and Copper ( II) Chloride Thermite Reaction 2Al(s)+Fe2O3(s) Al2O3(s)+2Fe(l) http://boyles.sdsmt.edu/thermite/ThirstrupThermit eClose.asx http://www2.chemie.unierlangen.de/education/medprak/videos/thermit_v. mpg http://video.google.com/videoplay?docid=7231843493488769585&q=Reactions&hl=en Aqueous Redox Reactions Oxidation States of Manganese Procedure Add 30 ml of a .01 M KMnO4 solution to four small flasks labeled A , B, N ( Place Tablet 1/10 ml water) To Flask A, Add 10 ml of 3M H2SO4 MnO4- + H+ To Flask B, add 10 ml of 5 M NaOH. MnO4- + OHTo Flask N add nothing. MnO4- Watch the color changes To Flask A add .01M NaHSO3 ( Tablet 2) slowly till you get a colorless Mn2+ ion. MnO4- + 5H++ HSO3- 3H2O + 2Mn2+ + 5SO42To Flask N add .01M NaHSO3 ( Tablet 2)until a brown precipitate forms. 2MnO4- + 3HSO33SO42- + H++ H2O +MnO2 To Flask B slowly add .01M NaHSO3 ( Tablet 2) until a green solution forms. 2MnO4- + OH-+ HSO32MnO42- + 2H2O + SO42- The Amazing Purple Drop Oil Drop Demo I2 + H20 HOI ( aq) + HI ( aq) Meanwhile I2 + 2e- = 2I-, Eo = 0.54 v HCHO + 2H+ + 2e- = CH3OH, Eo = 0.19 v Reactions Driven by Solubility and Precipitation Formation of Gases ( Increase in entropy) Formation of Water Coordinate Covalent Bond Formation ( Lewis Acid-Base) Formation Constants Formation of Water Metal Oxide + an Acid Salt + Water Metal Hydroxide + an Acid Salt + Water (a special type of reaction called neutralization) Milk of Magnesia Demo PREDICTIONS BASED ON SOLUBILITY If one or both of the products in the double replacement reaction is insoluble in water, the reaction will occur. Reaction #1 Lead Nitrate and Sodium Chromate Pb(NO3)2 (aq) + Na2CrO4 (aq) PbCrO4 (s) + Na NO3(aq) Pb 2+ + CrO42- PbCrO4 (s) Reaction # 2 Silver Nitrate and Hydrochloric Acid AgNO3(aq) + HCl(aq) AgCl (s) + HNO3 (aq) SOLUBILITY RULES FOR COMMON IONIC COMPOUNDS IN WATER 1. All nitrates, chlorates, and acetates are soluble in water. Silver acetate is sparingly soluble. 2. Most common acids are soluble in water. 3. All common IA, and ammonium compounds are soluble in water. 4. All chlorides, bromides, and iodides are soluble in water except silver, mercury (I), and lead. HgI2 and HgBr2 are insoluble in water. 5. All sulfates are soluble in water except CaSO4, SrSO4, BaSO4, PbSO4, Hg2SO4. Ag2SO4 is sparingly soluble in water. 6. All carbonates, phosphates, oxides, and sulfites are insoluble in water but soluble in dilute acids except the IA and ammonium compounds. 7. The sulfides of all metals are insoluble in water except the IA, IIA, and ammonium sulfides. 8. All hydroxides are insoluble in water except the IA, Ca(OH)2, Sr(OH)2, and Ba(OH)2 hydroxides. Combustion Whoosh Bottle Rocket Explosions Dynamite Soap Mixtures Repeating Exploding Flask