Gas Chromatography
advertisement

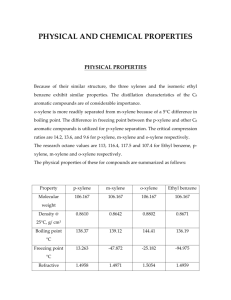
Introduction: In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic column.(2) Elution is brought about by the flow of an inert gaseous mobile phase.(2) The main reason why different compounds can be separated by the gas chromatography is the interaction of the compound with the stationary phase.(1) The stronger the interaction is the longer the compound remains attached to the stationary phase and the more time it takes to go through the column.(2) The purpose of this experiment is to separate the BTEX mixture by gas chromatographic techniques. In addition, the gas flow rate was the main discussed factor that affects the column efficiency. Observation: 1) Because the organic solvents which were used in this experiment such as Hexane and toluene are toxic, they were well stored and disposed in appropriate containers. Data: BTEX: benzene, toluene, ethylbenzene, and xylenes (o-xylene, m-xylene, p-xylene) in methanol. Column length: 25m Stationary phase: dimethylpolysiloxane Compound Hexane benzene toluene ethylbenzene p-xylene m-xylene o-xylene bromobenzene Boiling 69 80.1 110.6 136 138 139 144 156 point (°C) Discussion: To have a reasonable residence time in the column, a species must show some degree of compatibility with the stationary phase (1). The polarity of the stationary phase should match the sample components (1). In this experiment, the stationary phase dimethylpolysiloxane which is non-polar does not match the polar methanol. Therefore, even the sample compounds were dissolved in methanol, but the methanol was not eluted by the gas chromatography. Because of the boiling points of m-xylene and p-xylene are very close, the m-xylene and p-xylene peaks may overlap in the BTEX gas chromatogram. Furthermore, in the hexane chromatogram, there were more than one elution peak. This is because hexane has five isomers such as 2-methylpentane and 3-methylpentane. In addition, due to the similar structure, they have very close boiling point. Thus, some of the elution peaks may overlap as well in the chromatogram. The gas flow rate effects on column efficiency are measured by plate height H and plate count N. The efficiency of chromatographic columns increases as the plate count becomes greater and as the plate height becomes smaller. (1) Due to the Van Deemter plot, both of the very low and large flow rates will have large plate height which decreases the efficiency of chromatographic column. The retention factor is widely used to describe the migration rates of solutes on columns.(1) In this experiment, as the gas flow rate increases, the retention factor value increases, elution times become longer; however, while the flow rate was quick enough, the retention factor value decreases, elution times become shorter. Q1) Plot the chromatogram of the mixture. 50ppm BTEX at 55°C 30cm/s Q2) In this experiment, how do you identify which chromtographic peak corresponds to which compound? Suggest one method that is more reliable than this? In the gas chromatography, the chromatographic peaks can be identified by the boiling point of each compound. The low boiling point compound will be vaporized first and appeared chromatographic peak first. A better method here is to inject standard samples at the same temperature and velocity, and indentify the chromatographic peaks by comparing the retention time. Q3) Explain the elution order of these compounds. First, the order of elution is determined by the boiling point of the eluents. The order of the boiling point is hexane, benzene, toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, and bromobenzene (low to high). The lowest boiling point compound will be the first elution compounds. Thus, the elution order will be the same as the boiling point order. Q4) Plot and overlay the chromatograms at 7 flow rates. ’ Q5) calculate the tabulate k ,N and H for one compound for all runs. Sample calculation for o-xylene at 55°C 30cm/s N= 16tR2/W2= 16* 4.612/ 0.03952 = 217935.33 H= L/N = 2500cm / 217935.33 = 0.01147cm K’= (tR-tm)/tm = (4.61-2.324)/2.324= 0.98 o-xylene at 55°C N H K’ 10cm/s 132967.04 0.01880cm 0.62 15cm/s 72156.30 0.01609cm 2.24 20cm/s 181091.76 0.01381cm 2.22 25cm/s 210916.26 0.01185cm 1.68 30cm/s 217935.33 0.01147cm 0.98 40cm/s 224760.71 0.01112cm 0.37 50cm/s 295764.52 0.01428cm 0.37 Q6) Perform the Van Deemter plot and find out the optimal values of H and v. Van Deemter equation H= A+ B/u + Cu H(mm) 0.1880 0.1409 0.1381 0.1185 0.1147 0.1112 0.1428 V(cm/s) 10 15 20 25 30 40 50 Van Deemter Plot of Bromobenzene in 50ppm BTEX at 55C Plate Height (mm) 0.2 0.18 0.16 0.14 0.12 0.1 0 5 10 15 20 25 30 35 flow velority (cm/s) 40 45 50 55 Discussion: In this experiment, gas chromatography was used to analyze the BTEX mixture. The elution order depends on the boiling points of the analytes. The high boiling point component interacts longer with the stationary phase, therefore, the lowest boiling point analyte will be eluted and appear peak first.(1) The elution order of the BTEX mixture is benzene, toluene, ethylbenzene, p-xylene, m-xylene, and o-xylene (first-last). In this experiment, the flow rate effect is determined. In general, as the gas flow rate increases the column efficient and elution time increases. However, at very low or high gas flow rate, the column efficient and elution time is very low. Therefore, in order to control the characteristics of the gas chromatography, the infecting factor should be considered or try to keep them in constant. References: 1) SKOOG. HOLLER. NIEMAN. Principles of Instrumental Analysis, 5th ed. ; Brookes Cole; United States of America,1997;Vol.6, p 355-365. 2) Introductory Instrumental Analysis: Chemistry 316 Laboratory Manual; Simon Fraser University; Burnaby, 2010; p 13-21. 3) Wendy Strouse Watt, O.D. Fluorescein Angiogram, July, 2002. Gas Chromatography: Effect of column gas flow rate on isothermal GC separation of BTEX compounds MiaoMiao Gu 301100545 Chem 316 Monday, Nov. 1st,2010