Empirical Formula - Riverside Preparatory High School
advertisement

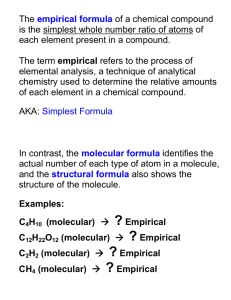
Homework Check • Find the percent composition of the following: 1. A compound with 222.6 g of Na and 77.4 g of O 2. Ammonium Nitrate (NH4NO3) 3. Ca(C2H3O2)2 4. If I had 124 g of Ca(C2H3O2)2, how many grams of Hydrogen would I have? Empirical Formula Percent to Formula • Molecular formula: Shows the number and kinds of atoms actually present H O 2 2 • Empirical formula: Gives the simplest ratio between atoms HO moles 4 Steps to Find the Empirical Formula 1. Replace the percent with grams 2. Convert grams to moles using the fence diagram 3. Divide the moles by the smallest number of the moles 4. If the ratio is not a whole number, then multiply to get a whole number mole ratio. (If the ratio is close to a whole number—0.1 away—then just round it off.) Rhymes to Remember Percent to Gram Gram to Mole Divide by Small Multiply till Whole Practice! 1. N = 36.8% O = 63.2% 2. C = 44.45% H = 6.21% O = 49.35% Telephone Game C = 46.15% H = 7.74% O = 46.11% Warm-Up! (Day 4) • Find the empirical formula of a compound that contains 40.9% carbon, 4.50% hydrogen, and 54.6% oxygen. • Molecular formula: Shows the number and kinds of atoms actually present H O 2 2 • Empirical formula: Gives the simplest ratio between atoms HO moles Steps to find the Molecular Formula 1. Calculate the molar mass of the empirical formula 2. Divide the actual molar mass by the empirical formula’s mass 3. Multiply the empirical formula’s subscripts by the answer from step #2 Practice! • A compound has a molar mass of 58.2 g/mol. If its empirical formula is C2H5, what is the molecular formula? Try these! (on a separate piece of paper) 1. What is the molecular formula that has a molar mass of 176 g/mol and its empirical formula is C3H4O3? 2. What is the molecular formula that has a molar mass of 194520 g/mol and its empirical formula is C6H10O5? Warm-Up! (Day 5) • Write the Rhymes for the empirical formula. Rhymes to Remember Percent to Gram Gram to Mole Divide by Small Multiply till Whole