Naming Binary Ionic Compounds
advertisement
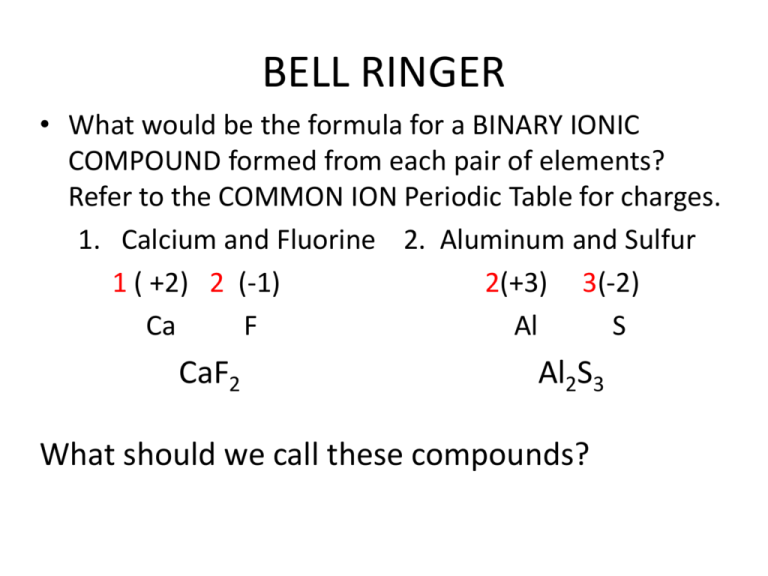
BELL RINGER • What would be the formula for a BINARY IONIC COMPOUND formed from each pair of elements? Refer to the COMMON ION Periodic Table for charges. 1. Calcium and Fluorine 2. Aluminum and Sulfur 1 ( +2) 2 (-1) 2(+3) 3(-2) Ca F Al S CaF2 Al2S3 What should we call these compounds? Naming Binary Ionic Compounds Binary Ionic Compounds • I CAN name a binary ionic compound from its properly written formula. What are BINARY Ionic Compounds? • The formulas we’ve written to this point represent BINARY IONIC compounds: – Consist of only TWO DIFFERENT ELEMENTS. – Contain a + METAL CATION and a – NONMETAL ANION. Naming a Binary Ionic Compound • To name a binary ionic compound: • 1. Name the + METAL ION first by its ELEMENT NAME. • 2. Name the – NON-METAL ION last by its ELEMENT NAME except: – Change the ENDING to “IDE”. • If the name ends in “INE” just switch to “IDE”. • If the name ends in something else, you may have to DROP PART OF THE NAME before changing to IDE. EXAMPLES • Chlorine – ends in “INE” – becomes CHLORIDE • What does Bromine become? BROMIDE • What about elements that do not end in INE? – Oxygen becomes OXIDE – Sulfur becomes SULFIDE – Phosphorus becomes PHOSPHIDE – Nitrogen becomes NITRIDE – Arsenic becomes ARSENIDE Practice Problems • Name these compounds: • • • • • NaCl CaF2 Ba3P2 K3N AlAs • • • • • SODIUM CHLORIDE CALCIUM FLUORIDE BARIUM PHOSPHIDE POTASSIUM NITRIDE ALUMINUM ARSENIDE More Practice Problems • Determine the FORMULA for the binary ionic compound formed by each pair of atoms, then NAME the compound. • 1. Rubidium and Fluorine 6. Francium and Oxygen • 2. Potassium and Phosphorus 7. Calcium and Bromine • 3. Radium and Nitrogen 8. Strontium and Arsenic • 4. Lithium and Sulfur 9. Aluminum and Selenium • 5. Boron and Fluorine 10. Rubidium and Astatine • 1. RbF • 2. K3P • 3. Ra3N2 Rubidium Fluoride Potassium Phosphide Radium Nitride • • • • • • • Lithium Sulfide Boron Fluoride Francium Oxide Calcium Bromide Strontium Arsenide Aluminum Selenide Rubidium Astatide 4. 5. 6. 7. 8. 9. 10. Li2S BF3 Fr2O CaBr2 Sr3As2 Al2Se3 RbAt




