H 2
advertisement

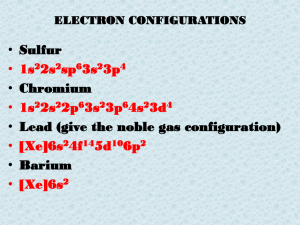
Copper (II) hydroxide Ionic HCl Acid Dinitrogen pentoxide Covalent Sodium Ionic chloride H2SO4 Acid Calcium hydroxide Ionic Carbon dioxide Covalent P 2 S5 Covalent Silver iodide Ionic Pb Lead Ca Calcium F Fluorine Hg Mercury Au Gold Cu Copper Mg Magnesium Mn Manganese Fe Iron When writing the formula for an ionic compound, the _____ is always written first. Cation (metal) Metals always ___ ____ electrons. Give away What is the ionic symbol for lithium? Li+1 What is the ionic symbol for sulfur? S-2 What is the ionic symbol for hydroxide? OH-1 What is the ionic symbol for ammonium? NH4+1 Covalent compounds are always between 2 _____. Nonmetals Naming covalent compounds includes using ___ before the element name. Prefixes What is the prefix for 1? Mono What is the prefix for 8? Octa What is the prefix for 5? 3? Penta, tri Calcium sulfate Ca+2 So4-2 = CaSO4 Potassium phosphate K+1 PO4-3 = K3PO4 Iron (II) chloride Fe+2 Cl-1 = FeCl2 lead (IV) hydroxide Pb+4 OH-1 = Pb(OH)4 Remember, the Roman numeral tells you the positive charge for the metal! Potassium chloride K+1 Cl-1 = KCl Magnesium fluoride Mg+2 F-1 = MgF2 Ammonium oxide NH4+1 O-2 = (NH4)2O S2O4 Disulfur tetroxide MgI2 Magnesium iodide AlBr3 Aluminum bromide CrCl3 Chromium (III) chloride NaOH Sodium hydroxide CaSO4 Calcium sulfate The law of conservation of mass and matter says matter cannot be created or destroyed! All chemical equations must, therefore, be balanced! Look at this formula: Pb(OH)4 There are ____ lead atoms, ____ oxygen atoms and ____ hydrogen atoms 1, 4,4 The formula Fe2(SO4)3 contains ____ Fe atoms, ___ S atoms and ___ O atoms 2, 3, 12 ___ H2 + ___ O2 → ___ H2O 2,1,2 ___ N2 +___ H2 → ___ NH3 1,3,2 ____Zn + ____ HCl → ___ ZnCl2 + ___ H2 1,2,1,1 ___ Fe + ___ O2 → ___ Fe2O3 4,3,2 THAT’S AS ALL FOR THE REVIEW!! ALWAYS, COME SEE ME IF YOU NEED SOME EXTRA HELP!!