Quiz Show Review of Simple Bonding Theory
Created by Carol J Breaux, College of the Ozarks, cbreaux@cofo.edu
and posted on
VIPEr (www.ionicviper.org
) on July 16, 2012, Copyright Carol J. Breaux, 2012. This work is licensed under the Creative Commons Non-Commercial Share Alike License. To view a copy of this license visit http://creativecommons.org/about/license/
Calculate the number of valence electrons available in an ion of CO
3
2.
24
26
18
20
22
Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are two for the charge.
Which of the following species is
NOT isoelectronic with the others?
SiF
2
CO
3
2-
NO
3
-
SO
3
BF
3
All have 24 valence electrons, except SiF
4
F has 7) which has 18 (Si has 4 and each
How many sigma and how many pi bonds in a molecule of HCN?
4 sigma, 0 pi
3 sigma, 0 pi
2 sigma, 2 pi
3 sigma, 1 pi
2 sigma, 1 pi
The CH bond is a sigma bond, one of the bonds from the CN triple bond is a sigma bond and the other two are pi bonds
What type of hybridization would exist around the central atom in NCl
3
?
sp 3 sp 2 sp sp 3 d s
There are three bonding pairs and one nonbonding pair on the nitrogen which will hybridize into 4 sp 3 hybrid orbitals
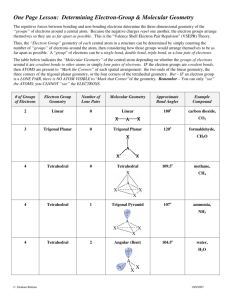
The electron geometry of nitrogen trichloride is
linear
Trigonal pyramid
Trigonal planar bent tetrahedral
Electron geometry includes the lone pair. There are four pairs of electrons around the central nitrogen giving a tetrahedral electron geometry
The expanded shell around the chlorine in ClF
3 contains how many electrons?
8
10
11
12
14
There are 28 valence electrons in this compound. The central Cl atom will have three bonding pairs of electrons to the fluorines and two lone pairs of electrons to accommodate all of the valence electrons
Increasing numbers of lone pairs on a central atom cause bond angles to decrease.
Electron pairs will repulse each other and will go as far apart from each other as they can. Lone pair-bonding pair repulsion is greater than bonding pair-bonding pair repulsion.
The molecular geometry of PCl
6
-
using VSEPR theory is
linear octahedral tetrahedral
Trigonal pyramidal
Trigonal planar
There are 48 valence electrons that can be accommodated by a central
P atom and 6 Cl atoms bonded octahedrally around the central atom. There are no lone pairs around the central P.
For which of the following compounds will the electron geometry and molecular geometry be the same?
NH
3
H
2
O
SF
4
CO
2
XeF
2
The electron geometry and the molecular geometry will be the same
For CO
2 because it is the only one that does not have any lone pairs on the central atom
A compound of type AX
4
E
2
which molecular geometry?
will have
tetrahedral
Square planar
Trigonal pyramid
Trigonal bipyramid octahedral
A stands for the central atom, X
4 atom, and E
2 is the number of bonds around the central is the number of lone pairs around the central atom. The lone pairs would occupy the axial positions and the 4 bonds would occupy the equatorial positions giving a square planar molecular geometry
The formal charge on the central atom in XeO
3
is
0
+1
+2
+3
-1
There are 26 valence electrons available for this compound. Xe is the central atom. There are three bonding pairs from the oxygens and one lone pair on the central atom. The free atom of Xe would have 8 valence electrons.
Subtract 2 for the lone pair electrons and 3 for each of the bonds from 8 and
The formal charge will be +3.
What would be the most likely hybridization for a molecule of PCl
5
?
sp 3 d 2 sp 3 dsp 2 sd 3 dsp 3
The 10 electrons in the expanded shell of P will hybridize from an s,
3 p orbitals, and one d orbital.
The occupancy or steric number on the central atom in a molecule of SF
4 is
5
4
3
2
1
The steric number or occupancy tells how many bonding pairs and lone pairs are around the central atom. The S has 4 bonding pairs from the 4 fluorines and one lone pair.