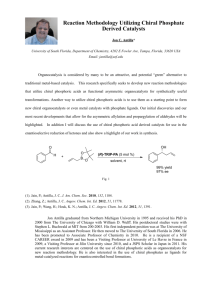
34. Enantioselective protonation
advertisement

Enantioselective protonation protonation as the enantio-determining step Brian M. Stoltz Nature Chemistry,2009,1,359 Song jin 2010-12-18 Gong group meeting Important factors in achieving enantioselective protonation ﹡ A fundamental method for generating a tertiary carbon stereocentre is to deliver a proton to a carbanion intermediate. H+ X Z Y ﹡ Principal: enantioselective protonations are necessarily kinetic processes, because under thermodynamic control a racemate would be formed. match the pKa of the proton donor and the product to prevent racemization before product isolation E transition state material product kinetic processes Approaches for non-enzymatic enantioselective protonation opportunities for asymmetric induction: 1.The use of a chiral Brønsted acid (Enantioselective protonation by a chiral proton donor) 2. Generation of a chiral protonacceptor intermediate (Enantioselective protonation by a chiral Brønsted base). 1.Chiral proton H+ X Z 2.Chiral carbanion intermediate Y Enantioselective protonation by a H+ X chiral proton donor 1.Chiral proton Y 2.Chiral carbanion intermediate Z 1.stoichiometric chiral proton source substrates :lithium enolates (a, b) a: Kim's enolate protonation OH OTMS CH3Li Et2O,CH2Cl2,-78℃ then cat O O Cl H CH3 Cl cat ﹡ A π–π-stacking interaction between the substrate and rigid proton source was proposed ee:3 J. Org. Chem. 2004, 69, 5104-5107 b: Eames's enolate protonation ee:69 enolates and proton sources can form an organized transition state that is guided by lithium chelation Bull. Chem. Soc. Jpn., 2005,78, 906–909 1.stoichiometric chiral proton source substrates : Enolate intermediates enolates accessed through decarboxylation(c) c: Rouden's decarboxylative protonation Proton donors : derived from Cinchona alkaloids alkaloid derivatives :dual-purpose reagents 1: they deprotonate malonate hemiester substrates and promote a decarboxylation event 2: the formed B*H served as a proton donor OL.2007,9,2621-2624 2. catalytic chiral proton source Stoichiometric to catalytic ? Choose an appropriate stoichiometric proton source a :Levacher's enol silane protonation ee:85 form a cinchonium fluoride ﹡ Deuterium labelling studies RCOF + R'OD O Me H(D) Conv>95% D%>95% ﹡ The fluoride anion of this active catalyst then activates the silyl group to facilitate proton delivery from the ammonium cation Angew. Chem. Int. Ed. 2007, 46, 7090 –7093 a :Levacher's enol silane protonation Past :using a ‘latent source of HF’ Now :using a carboxylic acid the same :activation silyl enolate 2 by means of fluoride or carboxylate anion. Snylett,2008,2447-2450 b: Yanagisawa's enol silane protonation ee:62 EtOH as a proton donor ee:14 i-PrOH as a proton donor ee:34 methanol as a proton donor ee:98 ee:97 ﹡the chiral Lewis acidic Ag∙BINAP complex binds methanol to generate a potent chiral Bronsted acid Angew. Chem. Int. Ed. 2005, 44, 1546 –1548 c. Yanagisawa's lithium enolate protonation A*H: 1.0eq A*H=1 A*H=proline ee:8-13 ee:1 BHT ee:76 -80 phenol ee:7 (S)-BINOL ee:9 2,2,6,6-tetramethyl-3,5-heptadione ee:56 ﹡ using a chiral Brønsted acid (0.1 equiv) in DMF at -78 °C for 5 min. Then, a solution of an achiral proton source (1 equiv) in THF was added (over a period of 2 h) at -78°C. OL. 2006, 8, 1721-1724 ﹡ Higher reactivity of A*-Li than that of the lithium enolate toward A-H is the key to success in the catalytic cycle. ﹡ both the catalyst 1 and BHT possess appropriate pKa values and BHT also has enough steric bulkiness to avoid the quick reaction with the lithium enolate, the catalytic cycle is considered to take place smoothly. Possible mechanism: HA* OH HA O OLi HA* 1.0eq OLi + * A*Li ee:8-10 OH OLi HA* + 0.1eq 0.9eq big enough to prevent racemic product racemate HA OLi + 0.1eq A*Li 0.1eq racemic product 0.1eq 1.0 eq HA slow addition over a period of 2 h A* O OLi O Li H O A O * + A*Li and ALi d: Yamamoto's enol silane protonation ﹡ In the absence of an achiral proton source, even though a stoichiometric amount of chiral Brønsted acid was used, no reaction was observed even after 2 days. Without PhOH, the desilylation was very slow because the affinity of the resultant conjugate base [A]- to the silicon is quite low. J. AM. CHEM. SOC. 2008, 130, 9246–9247 e: Rueping's quinoline reduction/protonation ﹡phosphoric acid: chiral proton souce Hantzch ester :an appropriate achiral stoichiometric proton source Adv. Synth. Catal. 2008, 350, 1001 – 1006 Organocatalytic Transfer Hydrogenation of Quinolines Angew. Chem. Int. Ed. 2006, 45, 3683 –3686 Mechanism: R N HO O P ArO OAr N H H R O O P ArO OAr H- (hydride ion) addition in the chiral center generation step Angew. Chem. Int. Ed. 2006, 45, 3683 –3686 Mechanism: + H addition in the chiral center generation step ﹡ Regeneration of the chiral proton source was made by using Hantzsch ester as the achiral proton source. Adv. Synth. Catal. 2008, 350, 1001 – 1006 f: Deng's conjugate addition/protonation ﹡ protonation for the generation of 1,3-stereocenters with excellent ee and dr ﹡ higher diastereoselectivity afforded by QD-1a than DABCO(dr =1.2:1) and other cinchona alkaloids ,the stereoselective protonation is due to catalyst control by QD-1a, not the substrate control by the quaternary stereocenter formed in the nuclephilic addition. J. AM. CHEM. SOC. 2006, 128, 3928-3930 Cinchona alkaloid derivative is proposed to serve two functions: 1.activating the Michael acceptor for addition 2.serving as the chiral Bronsted acid for the protonation of the nitrile-stabilized carbanion intermediate. g: Tan's conjugate addition/protonation Angew. Chem. Int. Ed. 2008, 47, 5641 –5645 h: Hénin/Muzart Norrish type II fragmentation/protonation loss of isobutylene ﹡ An unusual technique to generate an enol in situ Tetrahedrm , 1994, 50, 2849-2864. i: Fu's addition of hydrazoic acid to ketenes followed by Curtius rearrangement Curtius rearrangement the protonated catalyst serves as a chiral Bronsted acid Problem: rapid uncatalysed background reaction even at low temperatures, Solution: use sterically large ketenes Angew. Chem. Int. Ed. 2007, 46, 4367 –4369 Enantioselective protonation by a chiral Bronsted base ﹡ The use of nucleophilic heterocycle catalysts to generate chiral proton acceptors. a: Fu's addition of alcohols to ketenes chiral proton acceptors J. Am. Chem. Soc. 1999, 121, 2637-2638 ﹡ catalyst did not appreciably deprotonate methanol in solution. ﹡ catalyst did deprotonate HN3 in solution. ﹡ Cat Bronsted basicity :2<1 acidity of the [H-catalyst*]:2>1 ee <5% ee:96 b :Rovis' protonation of chloroenolates J. AM. CHEM. SOC. 2005, 127, 16406-16407 c :Scheidt's protonation of homoenolate equivalents ﹡ the site of protonation is relatively distant from the chiral control element. Synthesis. 2008,1306–1315 Conjugate addition/protonation sequences catalysed by chiral metal complexes a :Conjugate addition/protonation reactions catalysed by Rh•bis(phosphine) complexes Normal Mechanism: Ar ORh X Y Metal-catalysed 1,4-additions b :Divergent pathway consisting of β-hydride elimination, H-transfer, and protonation Angew. Chem. Int. Ed. 2004, 43, 719 –723 (Article) J. AM. CHEM. SOC. 2008, 130, 6159–6169 ﹡ Deuterium labelling studies ﹡ did not proceed via a direct protonation of the rhodium enolate Improved mechanism: Hydride transfer Chiral center generation β-hydride elimination (Article) J. AM. CHEM. SOC. 2008, 130, 6159–6169 1,2-addition c :Conjugate addition/protonation with a nitrogen nucleophile catalysed by palladium OL, 2005, 7, 2571-2573 d :Friedel–Crafts-type conjugate addition followed by enantioselective protonation Sibi . Angew. Chem. Int. Ed. 2008, 47, 9913 –9915 Transition metal-catalysed enantioselective protonation reactions by means of chiral metal enolates a :Trauner's Nazarov cyclization/enantioselective protonation ﹡ pericyclic reaction :A distinct method of accessing a chiral metal enolate a chiral metal enolate ﹡ R ≠ H, low diastereoselectivity low stereoselectivity in the electrocyclization step but high selectivity in the protonation step Dirk Trauner. J. AM. CHEM. SOC. 2004, 126, 9544-9545 Contrast reaction: Adv. Synth. Catal. 2009, 351, 78–84 b :Palladium-catalysed decarboxylative protonation reactions ﹡decarboxylation of β-ketoesters: a method accessing a chiral metal enolate. intercept this intermediate with an alternative electrophile, namely, a proton. Brian M. Stoltz J. AM. CHEM. SOC. 2004, 126, 15044-15045 J. AM. CHEM. SOC. 2006, 128, 11348-11349 c :Gadolinium-catalysed protonation reactions ﹡ the reaction proceeds via transmetallation from Si to Gd, and that this step is rate-limiting. ﹡ conjugate addition of cyanide to N-acryloyl pyrroles J. AM. CHEM. SOC. 2009, 131, 3858–3859 Conclusion ﹡ Useful transformations including natural products such as α- andβ-amino acids ﹡ Mechanistic understanding remains immature.