Adrenal cortical hormones 5
advertisement

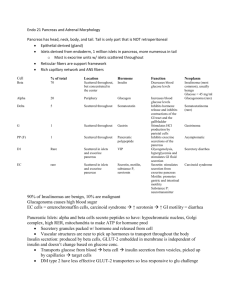
Adrenal cortical hormones By- Dr Shafali Singh Learning objectives • Layers of the adrenal cortex and their role in hormone synthesis • Biosynthetic pathways of steroid hormone synthesis and result of the enzyme deficiencies. • Physiological action of glucocorticoids and mineralocorticoids • Applied aspect Aldosterone and cortisol secretion are regulated by independent mechanisms Actions of Aldosterone ↑Aldosterone ↓Aldosterone • Hypokalemia - muscle weakness, • Hyperkalemia -cardiac arrhythmia ,mus paralysis • Metabolic alkalosis • Metabolic acidosis MINERALOCORTICOID DISORDERS Hyperaldosteronism with Hypertension with Hypotension LOW RENIN Primary hyperaldostero nism (Conn’s syndrome) HIGH RENIN Secondary hyperaldostero nism HIGH RENIN Secondary hyperaldosteronism Primary hyperaldosteronism (Conn’s syndrome) • Most common cause is a small unilateral adenoma, on either side • lncreased whole body sodium, fluid, and circulating blood volume • Edema rare (sodium escape*) • Blood pressure from borderline to severe hypertension • Modest left ventricular hypertrophy • weakness and fatigue. • Increased hydrogen ion excretion and new bicarbonate create metabolic alkalosis. • A positive Chvostek’s or Trousseau’s sign suggestive of alkalosis • Cortisol is normal. • Suppression of renin a major feature high BP + low renin = pri hyper aldo Secondary hyperaldosteronism with hypertension • In most cases a primary over-secretion of renin secondary to a decrease in renal blood flow and/or pressure • Renal arterial stenosis, narrowing via atherosclerosis, fibromuscular hyperplasia. • Modest to highly elevated renin • Modest to highly elevated aldosterone • Hypokalemia and metabolic alkalosis high BP+ High renin = renal artery stenosis Differential diagnosis: Hypokalemia in a hypertensive patient not taking diuretics • Hyposecretion of renin with elevated aldosterone that fails to respond to a volume contraction – Conn’s syndrome • Hypersecretion of renin with elevated aldosterone – renal artery atenosis Secondary hyperaldosteronism with hypotension • Cause--Sequestration of blood on the venous side of the systemic circulation. This results in decreased cardiac output and thus decreased blood flow and pressure in the renal artery. • causes: Congestive heart failure Constriction of the vena cava Hepatic cirrhosis nephrotic syndrome low B.P + high renin + high aldosterone =secondory hyp aldo Concerning Secondary Hyperaldosteronism with hypotension Aldosterone Secretion Increased Decreased • decreases pressure in the renal artery (e.g., hemorrhage, prolonged sweating) • High potassium intake • Low sodium intake • Constriction of inferior vena cava in thorax • Standing • Secondary hyperaldosteronism (in some cases of congestive heart failure, cirrhosis, and nephrosis) • increases blood pressure in the renal artery. This includes weightlessness, because blood no longer pools in the extremities when the individual is standing or sitting Consequences of the Loss of Regional Adrenal Function Zona glomerulosa: The absence of the mineralocorticoid, aldosterone, results in: • Loss of Na+ • Decreased volume of the ECF • Low blood pressure • Circulatory shock • Death (mineralocorticoid is generally required for survival) Hypocortisolism Primary Hypocortisolism (in primary adrenal insufficiency, Addison’s disease) Cortisol deficiency • leads to weakness, fatigue, anorexia, hypotension, hyponatremia, hypoglycemia. • Increases in ACTH result in hyperpigmentation of skin and mucous membranes. Aldosterone deficiency • leads to sodium wasting and hyponatremia, • Potassium retention • Hyperkalemia, dehydration, • Hypotension, and acidosis Androgen deficiency • Loss of axillary and pubic hair A) Tan and vitiligo. B) Pigmentation of scars from lesions that occurred after the development of the disease. C) Pigmentation of skin creases. D) Darkening of areolas. E) Pigmentation of pressure points. F) Pigmentation of the gums. • Excessive secretion of ACTH (e.g., Addison’s disease) causes darkening of the skin. • This is due to the melanocyte-stimulating hormone (α-MSH) sequence within the ACTH molecule, and the β-MSH activity of βlipotropin. _ Regulation of glucocorti coids secretion + _ _ + Transport, Metabolism, & Excretion of Adrenocortical Hormones Metabolic Actions of Cortisol • Effects on carbohydrate metabolismCortisol raises blood glucose, making more glucose available for nervous tissue. Two mechanisms are involved: cortisol inhibits glucose uptake in most tissues (muscle, lymphoid, and fat). cortisol increases hepatic output of glucose via gluconeogenesis from amino acids in particular (not from liver glycogenolysis) • Effects on protein metabolismCortisol promotes degradation and increased delivery of amino acids. Effects on lipid metabolism • ↑ Mobilisation of fatty acids from adipose tissue • ↑ FFA in plasma, ↑ oxidation of fat • Redistribution of fat – ‘Centripetal distribution’ • Utilises fat for energy • Potentiates lipolytic actions of GH, Glucagon, Catecholamines & Thyroxine Anti-inflammatory Action Decreases • capillary permeability • prostaglandin and leukotriene synthesis Disadvantage: As the glucocorticoids prevent inflammation, silent spread of infection occurs rapidly Immune supression Decreased production of • lymphocytes • interleukins 1 and 6 (IL-1 and IL-6) • T-cells Anti-allergic effect • Glucocorticoids prevent liberation of histamine from the mast cells by antigen-antibody complexes & prevent allergic manifestations. • Blocks inflammatory response to allergy Clinical uses : 1. Treatment of allergic diseases like eczema 2. Treatment of Bronchial Asthma Stress (Includes States Such as Trauma, Exposure to Cold, Illness and Exercise) Main agent to fight stress. Secretion of glucocorticoids increases during stress through activation of hypothalamopituitary-adrenal axis Resistance to stress • Stress - ↑ ACTH - ↑ cortisol - ↑ resistance • Rapid mobilisation of fat – energy changes Stress hormones (counter regulatory hormones) • G Growth hormone: mobilizes fatty acids by increasing lipolysis in adipose tissue • G Glucagon: mobilizes glucose by increasing liver glycogenolysis • G glucocorticoids;Cortisol (does not increase in starvation): mobilizes fat, protein, carbohydrate • Epinephrine, in some forms of stress such as exercise: mobilizes glucose via glycogenolysis and fat via lipolysis Effects on vascular system Effects on Mineral metabolism Effects on Nervous system: Effect on gasrtointestinal tract During foetal life Positive inotrophic By increasing the effect of catecholamines (Permissive Action ), it increases the smooth muscle tone & maintains B.P. Overlapping Action It increases Na+ store and deplete K+ like mineralocorticoids Calciuric Action modulate excitabilitybehavoiour and mood. Increase gastric acid secretion Decrease proliferation of gastric mucosal cells Maturation of lungs,gastro intestinal tract,CNS, retina ,skin Permissive Actions of Cortisol Glucagon • Promotes glycogenolysis in the liver (some lipolysis from adipocytes as well). • Without cortisol, fasting hypoglycemia rapidly develops. Catecholamines • Promote glycogenolysis and lipolysis in liver and muscle. Promote vasoconstriction and bronchodilation. • Without cortisol, blood pressure decreases. Hypercortisolism Primary (Adrenal adenoma – Benign, unilateral) Secondary( High ACTH) 1. Pituitary –cushing disease 2.Ectopic ACTH production Hypocortisolism Primary (Adrenal insufficiencyAddison’s disease) Secondary( Low ACTH) 1. Exogenous cortisol withdrawl 2. Pituitary tumor Glucocorticoid Excess • Cushing syndrome: hypercortisolism regardless of origin, including chronic glucocorticoid therapy • Cushing disease: hypercortisolism due to an adenoma of the anterior pituitary (microadenoma) Excessive cortisol • Muscle weakness (excessive proteolysis ; hypokalemia (via mineralocorticoid actions), • Skin thins and is more readily damaged(Cortisol inhibits fibroblast proliferation and collagen formation). • The connective tissue support of capillaries is impaired, and capillary injury, or bruising, is increased . • Incr Glucose • Redistribution of Fat • Incr B.P • Euphoria Suppression Tests 1. Low-dose dexamethasone (for the presence of Cushing syndrome) cortisol decreases Normal cortisol not suppressed Hypercortisolism; 2. High-dose dexamethasone( To differentiate Cushing disease from ectopic ACTH secretion and adrenal tumors) cortisol decreases Cushing disease; cortisol not suppressed Ectopic ACTH, or adrenal tumor; Metyrapone testing • Mainly to access pituitary-adrenal reserve • Inhibits 11 beta hydroxylase, thereby decreases cortisol • Normal pituitary ; ACTH increases, 11deoxycortisol increases • ACTH level no change, pituitary not functioning Hypercortisolism Primary hypercortisolism (adrenal origin) • Cortisol elevated, ACTH depressed • Most are benign adrenocortico adenomas • Adrenal adenoma usually unilateral and secretes only cortisol; decreased adrenal androgen and deoxycorticosterone (hirsutism absent) Presence of androgen or mineralocorticoid excess suggests a carcinoma. Secondary hypercortisolism (pituitary/ectopic) • ACTH dependent • Elevated ACTH, cortisol, adrenal androgen, deoxycorticosterone • Hypersecretion of ACTH results in bilateral hyperplasia of the adrenal zona fasciculata and reticularis Cushing disease Ectopic ACTH syndrome • Cause is a pituitary adenoma usually a microadenoma. • Most common pathological cause of Cushing syndrome • Increased ACTH not sufficient to cause hyperpigmentation • Dexamethasone suppressible • Most frequently in patients with small cell carcinoma of the lung • Greater secretion of ACTH than in Cushing disease and hyperpigmentation often present • Ectopic site nonsupressible with dexamethasone • Typical features of Cushing syndrome often absent due to malignancy Hypocortisolism Primary Hypocortisolism (in primary adrenal insufficiency, Addison’s disease) Cortisol deficiency • leads to weakness, fatigue, anorexia, hypotension, hyponatremia, hypoglycemia. • Increases in ACTH result in hyperpigmentation of skin and mucous membranes. Aldosterone deficiency • leads to sodium wasting and hyponatremia, • Potassium retention • Hyperkalemia, dehydration, • Hypotension, and acidosis Androgen deficiency • Loss of axillary and pubic hair Absence of the glucocorticoid, cortisol, The contributes to: • Circulatory failure, because without cortisol, catecholamines do not exert their normal vasoconstrictive action • An inability to readily mobilize energy sources (glucose and free fatty acids) from glycogen or fat. • Under normal living conditions, this is not lifethreatening; however, under stressful situations, severe problems can arise. For example, fasting can result in fatal hypoglycemia. A) Tan and vitiligo. B) Pigmentation of scars from lesions that occurred after the development of the disease. C) Pigmentation of skin creases. D) Darkening of areolas. E) Pigmentation of pressure points. F) Pigmentation of the gums. Q The signs and symptoms of a patient with primary adrenal insufficiency include a. Pallor b. Low ACTH levels c. High cortisol levels d. Hyperkalemia e. Hypertension Addisonian crisis: Minor stress precipitates collapse • After adrenalectomy • Abrupt withdrawal of cortisol • Treatment: IV cortisol, glucose, NaCl Maintenance: lifetime cortisol & aldosterone Stimulation Tests • Rapid ACTH stimulation test • To diagnose both primary and secondary hypocortisolism (atrophied adrenal nonresponsive) • Normal; cortisol increases • Primary Hypocortisolism; cortisol no change Secondary hypocortisolism(central) • Most commonly due to sudden withdrawal of exogenous glucocorticoid therapy • Pituitary or hypothalamic tumors most common natural origin of ACTH deficiency • May be associated with the loss of other anterior pituitary hormones (pan-hypopituitarism) or adenomas secreting prolactin or growth hormone • Atrophy of the zona fasciculata and zona reticularis • Zona glomerulosa and aldosterone normal; no manifestations of miner-alocorticoid deficiency When prolonged treatment with anti-inflammatory doses of glucocorticoids is stopped adrenal is atrophic and unresponsive after cortisol treatment, pituitary unable to secrete normal amounts of ACTH for as long as a month. Thereafter, ACTH secretion slowly increases to supranormal levels. These in turn stimulate the adrenal, and glucocorticoid output rises, with feedback inhibition gradually reducing the elevated ACTH levels to normal .. Primary and Secondary Disorders of Cortisol Secretion Q. Cortisol administration to a patient with adrenal insufficiency will a. Increase insulin sensitivity in muscle b. Enhance wound healing c. Increase corticotropin-releasing hormone secretion d. Increase ACTH secretion e. Increase gluconeogenesis ADRENAL ANDROGENS • Major-DHEA, Androstenedione Minor-oestrogen,progestrone Androgens are the hormones that exert masculinizing effects and they promote protein anabolism and growth • • • • Actions: Less than 20% of testosterone activity Little masculinising effect Early development of male sex organs Growth of pubic hair, axillary hair Control: • By ACTH • Medulla: The absence of the catecholamine, epinephrine (the major hormone of the adrenal medulla), results in: • Decreased capacity of the individual to mobilize glycogen or fat during exercise or cold exposure; • however, the adrenal medulla is not essential for survival • Synthesis and Secretion of Adrenocortical Hormones Pathway to aldosterone synthesis Control of Steroid Hormone Synthesis in the Zonas Fasciculata and Reticularis C19 steroids (19 carbon atoms) Adrenal Androgens • Have a keto group at position 17 and are therefore called 17-ketosteroids. • DHEA, DHEA sulfate, and androstenedione have very low androgenic activity. They function primarily as precursors for the peripheral conversion to the more potent testosterone and dihydrotestosterone (men and women). • In adult males, excessive production of adrenal androgens has no clinical Consequences. • In prepubertal males it causes premature penile enlargement and early development of secondary sexual characteristics. • In women excessive adrenal androgens cause hirsutism and virilization. Adrenal androgen • Are conjugated with sulfate in the adrenal cortex, making them water soluble. • As water-soluble metabolites, they circulate in the bloodstream, are filtered by the kidney, and are excreted in the urine. • The sulfated form is not produced in the gonads and is thus considered an index of androgen production by the adrenals Urinary 17-ketosteroids Are an index of all androgens, adrenal and testicular. In females and prepubertal males, urinary 17ketosteroids are an index of adrenal androgen secretion. In adult males (postpuberty), urinary 17ketosteroids are 2/3 adrenal and 1/3 testicular, and thus mainly an index of adrenal secretion. Which step in steroid hormone biosynthesis is stimulated by adrenocorticotropic hormone (ACTH)? (A) Cholesterol → pregnenolone (B) Progesterone → 11-deoxycorticosterone (C) 17-Hydroxypregnenolone → dehydroepiandrosterone (D) Testosterone → estradiol (E) Testosterone → dihydrotestosterone Which step in steroid hormone biosynthesis, if inhibited, blocks the production of all androgenic compounds but does not block the production of glucocorticoids? (A) Cholesterol → pregnenolone (B) Progesterone → 11-deoxycorticosterone (C) 17-Hydroxypregnenolone → dehydroepiandrosterone (D) Testosterone → estradiol (E) Testosterone → dihydrotestosterone Enzyme Deficiencies • Single enzyme defects can occur as congenital “inborn errors of metabolism.” • Congenital defects in any of the enzymes lead to deficient cortisol secretion and the syndrome called congenital adrenal hyperplasia. 21 β-Hydroxylase Deficiency Summary of overall pathway changes: • Zona glomerulosa: decreased aldosterone • : decreased production of 11-deoxycorticosterone, a weak mineralocorticoid. • Therefore, a mineralocorticoid deficiency, loss of Na+, volume and a hypotensive state. • Increased renin secretion and increased circulating angiotensin II. • Decreased production of corticosterone, a weak glucocorticoid, and cortisol. • Therefore, glucocorticoid deficiency and increased ACTH, which drive increases in adrenal androgen secretion 21 β-Hydroxylase deficiency cont.. • Accounts for about 90% of the cases • Neonates may present with a salt-wasting crisis. • Salt wasters tend to have hyponatremia, hyperkalemia, and raised plasma renin. • 17-hydroxyprogesterone is elevated. • Increased androgens lead to virilization of the female fetus and sexual ambiguity at birth • Males are phenotypically normal at birth but develop precocious pseudopuberty, growth acceleration, premature epiphyseal plate closure, and diminished final height. • Goal in treatment is to bring glucocorticoid and mineralocorticoid back to the normal range which would also suppress adrenal androgen secretion. 11 β-Hydroxylase Deficiency Summary of overall pathway changes: decreased corticosterone and cortisol, increased ACTH and overproduction of steroids above the blockade, including: • – Androgens and the consequences in women and prepubertal males • – 11-deoxycorticosterone, a mineralocorticoid that leads to hypertension and a decrease in circulating angiotensin II Zona glomerulosa: decreased stimulation of the steroid pathway and aldosterone production due to the hypertensive decrease in circulating angiotensin II 11 β-Hydroxylase deficiency Accounts for about 7% of all cases • Clinical features of increased androgens similar to the preceding form, including virilization of female fetus. • The principal difference with this form is the hypertension produced by 11 deoxycorticosterone, along with hypokalemia and suppressed renin secretion. • Milder forms do not present with hypertension and its consequences. 17 α-Hydroxylase Deficiency Effect in the testes Effect in the ovaries • Zona fasciculata, reticularis: decreased adrenal androgens, decreased cortisol, and increased ACTH. Increased 11-deoxycorticosterone leading to hypertension. • The reduced circulating angiotensin II reduces stimulation of zona glomerulosa and aldosterone secretion • Extremely rare • no sex hormones are produced, so female external genitalia are present. • Usually diagnosed at the time of puberty when the patient presents with hypertension, hypokalemia, and hypogonadism • Individuals have eunuchoid characteristics Summary of enzyme deficiency • Cholesterol desmolase deficiency is fatal in utero because it prevents the placenta from making the progesterone necessary for pregnancy to continue. • Mutation of the gene for the steroidogenic acute regulatory (StAR) protein A cause of severe congenital adrenal hyperplasia in newborns. This protein is required for the normal movement of cholesterol into the mitochondria to reach cholesterol desmolase. • A new born female is hypotensive and hypoactive .She also has a labial fusion and clitromegaly .The prenatal period and delivery were uncomplicated .Laboratory investigations reveal increased urinary 17hydroxyprogesterone exceretion and decreased 11 – deoxycortisone excretion. Which of the following enzymes is most likely to be deficient in this patient? • • • • • 17- hydroxylase 21- hydroxylase 11- hydroxylase Desmolase 5 α reductase Which step in steroid hormone biosynthesis occurs in the accessory sex target tissues of the male and is catalyzed by 5α-reductase? (A) Cholesterol → pregnenolone (B) Progesterone → 11-deoxycorticosterone (C) 17-Hydroxypregnenolone → dehydroepiandrosterone (D) Testosterone → estradiol (E) Testosterone → dihydrotestosterone Q. If a heart transplant patient receives prednisone (a glucocorticoid) to help prevent rejection of the transplanted tissue, will blood levels of ACTH and CRH be high or low? Q The secretion of ACTH is correctly described in which of the follow-ing statements? • a. It shows circadian rhythm in humans • b. It is decreased during periods of stress • c. It is inhibited by aldosterone • d. It is stimulated by glucocorticoids • e. It is stimulated by epinephrine