Ch12 Temperature and Heat Common Temperature Scales
advertisement

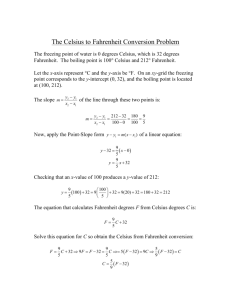
Ch12. Temperature and Heat Common Temperature Scales A number of different temperature scales have been devised, two popular choices being the Celsius (formerly, centigrade) and Fahrenheit scales. 1 On the Celsius scale, an ice point of 0 °C (0 degrees Celsius) and a steam point of 100 °C were selected. On the Fahrenheit scale, an ice point of 32 °F (32 degrees Fahrenheit) and a steam point of 212 °F were chosen. The Celsius scale is used worldwide, while the Fahrenheit scale is used mostly in the United States. The temperature of the human body is about 37 °C, where the symbol °C stands for “degrees Celsius.” However, the change between two temperatures is specified in “Celsius degrees ”(C°)—not in “degrees Celsius”. The separation between the ice and steam points on the Celsius scale is divided into 100 Celsius degrees, while on the Fahrenheit scale the separation is divided into 180 Fahrenheit degrees. Therefore, the size of the Celsius degree is larger than that of the Fahrenheit degree by a factor of 180 , or 9 . 2 100 5 Example 1. Converting from a Fahrenheit to a Celsius Temperature A healthy person has an oral temperature of 98.6 °F. What would this reading be on the Celsius scale? Reasoning and Solution A temperature of 98.6 °F is 66.6 Fahrenheit degrees above the ice point of 32.0 °F. Since , the difference of 66.6 F° is equivalent to Thus, the person’s temperature is 37.0 Celsius degrees above the ice point. Adding 37.0 Celsius degrees to the ice point of 0 °C on the Celsius scale gives a Celsius temperature of 3 Example 2. Converting from a Celsius to a Fahrenheit Temperature A time and temperature sign on a bank indicates that the outdoor temperature is –20.0 °C. Find the corresponding temperature on the Fahrenheit scale . The temperature, then, is 36.0 Fahrenheit degrees below the ice point. Subtracting 36.0 Fahrenheit degrees from the ice point of 32.0 °F on the Fahrenheit scale gives a Fahrenheit temperature of 4 Reasoning Strategy Converting Between Different Temperature Scales 1. Determine the magnitude of the difference between the stated temperature and the ice point on the initial scale. 2. Convert this number of degrees from one scale to the other scale by using the fact that. 3.Add or subtract the number of degrees on the new scale to or from the ice point on the new scale. 5 Check Your Understanding 1 On a new temperature scale the steam point is 348 °X, and the ice point is 112 °X. What is the temperature on this scale that corresponds to 28.0 °C? 178 °X 6 The Kelvin Temperature Scale Kelvin temperature scale was introduced by the Scottish physicist William Thompson (Lord Kelvin, 1824–1907), and in his honor each degree on the scale is called a kelvin (K). By international agreement, the symbol K is not written with a degree sign (°), nor is the word “degrees” used when quoting temperatures. For example, a temperature of 300 K (not 300 °K) is read as “three hundred kelvins,” not “three hundred degrees kelvin.” The kelvin is the SI base unit for temperature. 7 The ice point (0 °C) occurs at 273.15 K on the Kelvin scale. When a gas confined to a fixed volume is heated, its pressure increases. Conversely, when the gas is cooled, its pressure decreases. The change in gas pressure with temperature is the basis for the constant-volume gas thermometer. 8 A constant-volume gas thermometer. 9 A plot of absolute pressure versus temperature for a low-density gas at constant volume. The graph is a straight line and, when extrapolated (dashed line), crosses the temperature axis at –273.15 °C. “Absolute zero” means that temperatures lower than – 273.15 °C cannot be reached by continually cooling a gas or any other substance. 10 Thermometers A property that changes with temperature is called a thermometric property. The thermocouple is a thermometer used extensively in scientific laboratories. It consists of thin wires of different metals, welded together at the ends to form two junctions. 11 12 One of the junctions, called the “hot” junction, is placed in thermal contact with the object whose temperature is being measured. The other junction, termed the “reference” junction, is kept at a known constant temperature (usually an ice–water mixture at 0 °C). The thermocouple generates a voltage that depends on the difference in temperature between the two junctions. This voltage is the thermometric property and is measured by a voltmeter. 13 Because this electrical resistance changes with temperature, electrical resistance is another thermometric property. Electrical resistance thermometers are often made from platinum wire, because platinum has excellent mechanical and electrical properties in the temperature range from –270 °C to +700 °C. The electrical resistance of platinum wire is known as a function of temperature. Thus, the temperature of a substance can be determined by placing the resistance thermometer in thermal contact with the substance and measuring the resistance of the platinum wire. 14 Radiation emitted by an object can also be used to indicate temperature. At low to moderate temperatures, the predominant radiation emitted is infrared. As the temperature is raised, the intensity of the radiation increases substantially. “Thermal painting” is called a thermograph or thermogram. Thermography is an important diagnostic tool in medicine. 15 Linear Thermal Expansion NORMAL SOLIDS The increase in any one dimension of a solid is called linear expansion . When the temperature of a rod is raised by T, the length of the rod increases by L . 16 For modest temperature changes, experiments show that the change in length is directly proportional to the change in temperature L T In addition, the change in length is proportional to the initial length of the rod. L is proportional to both L0 and T ( L L0 T ) by using a proportionality constant , which is called the coefficient of linear expansion. 17 LINEAR THERMAL EXPANSION OF A SOLID The length L0 of an object changes by an amount L when its temperature changes by an amount T: L L0 T where is the coefficient of linear expansion. Common Unit for the Coefficient of Linear Expansion: 1 1 C C 18 Coefficient of Thermal Expansion (C°)–1 Substance Linear (a) Volumetric (b) Solids 23 × 10–6 69 × 10–6 Brass 19 × 10–6 57 × 10–6 Concrete 12 × 10–6 36 × 10–6 Copper 17 × 10–6 51 × 10–6 Glass (common) 8.5 × 10–6 26 × 10–6 Aluminium 3.3 × 10–6 9.9 × 10–6 Gold 14 × 10–6 42 × 10–6 Iron or steel 12 × 10–6 36 × 10–6 Lead 29 × 10–6 87 × 10–6 Nickel 13 × 10–6 39 × 10–6 Quartz (fused) 0.50 × 10–6 1.5 × 10–6 Silver 19 × 10–6 Glass (Pyrex) 57 × 10–6 19 Liquidsb Benzene — 1240 × 10–6 Carbon tetrachloride — 1240 × 10–6 Ethyl alcohol — 1120 × 10–6 Gasoline — 950 × 10–6 Mercury — 182 × 10–6 Methyl alcohol — 1200 × 10–6 Water — 207 × 10–6 20 Example 3. Buckling of a Sidewalk 21 A concrete sidewalk is constructed between two buildings on a day when the temperature is 25 °C. The sidewalk consists of two slabs, each three meters in length and of negligible thickness . As the temperature rises to 38 °C, the slabs expand, but no space is provided for thermal expansion. The buildings do not move, so the slabs buckle upward. Determine the vertical distance y in part b of the drawing. 22 Antiscalding device screws onto the end of a faucet and quickly shuts off the flow of water when it becomes too hot. As the water temperature rises, the actuator spring expands and pushes the plunger forward, shutting off the flow. When the water cools, the spring contracts and the water flow resumes. 23 THERMAL STRESS: Example 4. The Stress on a Steel Beam A steel beam is used in the roadbed of a bridge. The beam is mounted between two concrete supports when the temperature is 23 °C, with no room provided for thermal expansion. What compressional stress must the concrete supports apply to each end of the beam, if they are to keep the beam from expanding when the temperature rises to 42 °C? 24 F L Stress Y A L0 L0 T L Stress Y Y YT L0 L0 Y = 2.0 × 1011 N/m2 = 12 × 10–6 (C°) –1 T = 19 C° 25 THE BIMETALLIC STRIP A bimetallic strip is made from two thin strips of metal that have different coefficients of linear expansion. Bass Steel 26 Bimetallic strips are frequently used as adjustable automatic switches in electrical appliances. 27 28 THE EXPANSION OF HOLES: Conceptual Example 5. Do Holes Expand or Contract When the Temperature Increases? Eight square tiles that are arranged to form a square pattern with a hole in the center. If the tiles are heated, what happens to the size of the hole? 29 The hole expands just as if it were made of the material of the surrounding tiles. 30 Example 6. A Heated Engagement Ring A gold engagement ring has an inner diameter of 1.5 × 10–2 m and a temperature of 27 °C. The ring falls into a sink of hot water whose temperature is 49 °C. What is the change in the diameter of the hole in the ring? = 14 × 10–6 (C°)–1 31 Conceptual Example 7. Expanding Cylinders In a cross-sectional view of three cylinders, A, B, and C, each is made from a different material: one is lead, one is brass, and one is steel. All three have the same temperature, and they barely fit inside each other. As the cylinders are heated to the same, but higher, temperature, cylinder C falls off, while cylinder A becomes tightly wedged to cylinder B. Which cylinder is made from which material? 32 A = brass, B = steel, and C = lead A = lead, B = steel, and C = brass 33 Check Your Understanding 2 A metal ball has a diameter that is slightly greater than the diameter of a hole that has been cut into a metal plate. The coefficient of linear thermal expansion for the metal from which the ball is made is greater than that for the metal of the plate. Which one or more of the following procedures can be used to make the ball pass through the hole? (a)Raise the temperatures of the ball and the plate by the same amount. (b)Lower the temperatures of the ball and the plate by the same amount. (c) Heat the ball and cool the plate. (d)Cool the ball and heat the plate. b&d 34 Volume Thermal Expansion VOLUME THERMAL EXPANSION The volume V0 of an object changes by an amount V when its temperature changes by an amount T: V V0 T where is the coefficient of volume expansion. Common Unit for the Coefficient of Volume Expansion: (C°) –1 = 3. 35 Example 8. An Automobile Radiator A small plastic container, called the coolant reservoir, catches the radiator fluid that overflows when an automobile engine becomes hot . The radiator is made of copper, and the coolant has a coefficient of volume expansion of . If the radiator is filled to its 15-quart capacity when the engine is cold (6.0 °C), how much overflow from the radiator will spill into the reservoir when the coolant reaches its operating temperature of 92 °C? 36 V V0T 4.10 10 C 1 4 15quarts86C 0.53quarts 5110 6 C 1 V V0T 5110 C 6 1 15quarts86C 0.066quarts The overflow volume is 0.53 quarts – 0.066 quarts = 0.46 quarts. 37 The fact that water has its greatest density at 4 °C, rather than at 0 °C, has important consequences for the way in which a lake freezes. 38 The fact that the density of ice is smaller than the density of water has an important consequence for home owners, who have to contend with the possibility of bursting water pipes during severe winters. 39 Heat and Internal Energy Heat is energy in transit from hot to cold. (a)Heat flows from the hotter coffee cup to the colder hand. (b)Heat flows from the warmer hand to the colder glass of ice water. 40 DEFINITION OF HEAT Heat is energy that flows from a higher-temperature object to a lower-temperature object because of the difference in temperatures. SI Unit of Heat: joule (J) The internal energy of a substance is the sum of the molecular kinetic energy (due to the random motion of the molecules), the molecular potential energy (due to forces that act between the atoms of a molecule and between molecules), and other kinds of molecular energy. When heat flows in circumstances where the work done is negligible, the internal energy of the hot substance decreases and the internal energy of the cold substance increases. 41 42 Heat and Temperature Change: Specific Heat Capacity SOLIDS AND LIQUIDS HEAT SUPPLIED OR REMOVED IN CHANGING THE TEMPERATURE OF A SUBSTANCE The heat Q that must be supplied or removed to change the temperature of a substance of mass m by an amount T is Q cmT where c is the specific heat capacity of the substance. Common Unit for Specific Heat Capacity: J/(kg·C°) 43 Substance Specific Heat Capacity, c J/(kg·C°) Solids Aluminum 9.00 × 102 Copper 387 Glass 840 Human body (37 °C, average) 3500 Ice (–15 °C) 2.00 × 103 Iron or steel 452 Lead 128 Silver 235 44 Liquids Benzene 1740 Ethyl alcohol 2450 Glycerin 2410 Mercury 139 Water (15 °C) 4186 45 Example 9. A Hot Jogger In a half hour, a 65-kg jogger can generate 8.0 × 105 J of heat. This heat is removed from the jogger’s body by a variety of means, including the body’s own temperatureregulating mechanisms. If the heat were not removed, how much would the body temperature increase? 46 Example 10. Taking a Hot Shower Cold water at a temperature of 15 °C enters a heater, and the resulting hot water has a temperature of 61 °C. A person uses 120 kg of hot water in taking a shower. (a) Find the energy needed to heat the water. (b) Assuming that the utility company charges $0.10 per kilowatt·hour for electrical energy, determine the cost of heating the water. (a ) 47 (b) At a cost of $0.10 per kWh, the bill for the heat is $0.64 or 64 cents. 48 GASES The value of the specific heat capacity depends on whether the pressure or volume is held constant while energy in the form of heat is added to or removed from a substance. The distinction between constant pressure and constant volume is usually not important for solids and liquids but is significant for gases. 49 HEAT UNITS OTHER THAN THE JOULE There are three heat units other than the joule in common use. One kilocalorie (1 kcal) was defined historically as the amount of heat needed to raise the temperature of one kilogram of water by one Celsius degree. one calorie (1 cal) was defined as the amount of heat needed to raise the temperature of one gram of water by one Celsius degree The British thermal unit (Btu) is the other commonly used heat unit and was defined historically as the amount of heat needed to raise the temperature of one pound of water by one Fahrenheit degree. 50 Joule’s experiments revealed that the performance of mechanical work, like rubbing your hands together, can make the temperature of a substance rise, just as the absorption of heat can. This conversion factor is known as the mechanical equivalent of heat. 51 CALORIMETRY The kind of heat transfer that occurs within a thermos of iced tea also occurs within a calorimeter, which is the experimental apparatus used in a technique known as calorimetry. 52 Example 11. Measuring the Specific Heat Capacity A calorimeter cup is made from 0.15 kg of aluminum and contains 0.20 kg of water. Initially, the water and the cup have a common temperature of 18.0 °C. A 0.040-kg mass of unknown material is heated to a temperature of 97.0 °C and then added to the water. The temperature of the water, the cup, and the unknown material is 22.0 °C after thermal equilibrium is reestablished. Ignoring the small amount of heat gained by the thermometer, find the specific heat capacity of the unknown material. 53 (cmT ) Al (cmT ) water (cmT )unknown Heat gained by aluminum and water Heat lost by unknown material c Al m Al TAl cwater mwater Twater cunknown munknownTunknown 54 TAl = Twater = 22.0 °C – 18.0 °C = 4.0 C° Tunknown = 97.0 °C – 22.0 °C = 75.0 C°. 55 Check Your Understanding 3 Consider a mass m of a material and a change T in its temperature. Various possibilities for these variables are listed in the table below. Rank these possibilities in descending order (largest first), according to how much heat is needed to bring about the change in temperature. m (kg) c, b, d, a T (C°) (a) 2.0 15 (b) 1.5 40 (c) 3.0 25 (d) 2.5 20 56 Heat and Phase Change: Latent Heat Three familiar phases of matter—solid, liquid, and gas—and the phase changes that can occur between any two of them. 57 The graph shows the way the temperature of water changes as heat is added, starting with ice at –30 °C. The pressure is atmospheric pressure. 58 Conceptual Example 12. Saving Energy Suppose you are cooking spaghetti for dinner, and the instructions say “boil the pasta in water for ten minutes.” To cook spaghetti in an open pot with the least amount of energy, should you turn up the burner to its fullest so the water vigorously boils, or should you turn down the burner so the water barely boils? Turn down the heat, because the least amount of energy is expended when the water barely boils. 59 HEAT SUPPLIED OR REMOVED IN CHANGING THE PHASE OF A SUBSTANCE The heat Q that must be supplied or removed to change the phase of a mass m of a substance is where L is the latent heat of the substance. SI Unit of Latent Heat: J/kg The latent heat of fusion Lf refers to the change between solid and liquid phases, the latent heat of vaporization Lv applies to the change between liquid and gas phases, and the latent heat of sublimation Ls refers to the change between solid and gas phases. 60 Substance Melting Point (°C) Latent Heat of Fusion, Lf (J/kg) Boiling Point (°C) Latent Heat of Vaporization, Lv (J/kg) Ammonia –77.8 33.2 × 104 –33.4 13.7 × 105 Benzene 5.5 12.6 × 104 80.1 3.94 × 105 Copper 1083 20.7 × 104 2566 47.3 × 105 Ethyl alcohol –114.4 10.8 × 104 78.3 8.55 × 105 Gold 1063 6.28 × 104 2808 17.2 × 105 Lead 327.3 2.32 × 104 1750 8.59 × 105 Mercury –38.9 1.14 × 104 356.6 2.96 × 105 Nitrogen –210.0 2.57 × 104 –195.8 2.00 × 105 Oxygen –218.8 1.39 × 104 –183.0 2.13 × 105 Water 0.0 33.5 × 104 100.0 22.6 × 105 61 Example 13. Ice-cold Lemonade Ice at 0 °C is placed in a Styrofoam cup containing 0.32 kg of lemonade at 27 °C. The specific heat capacity of lemonade is virtually the same as that of water; that is, c = 4186 J/(kg·C°). After the ice and lemonade reach an equilibrium temperature, some ice still remains. The latent heat of fusion for water is Lf = 3.35 × 105 J/kg. Assume that the mass of the cup is so small that it absorbs a negligible amount of heat, and ignore any heat lost to the surroundings. Determine the mass of ice that has melted. 62 Q = cm T Q = mLf mL f ice cm T Heat gained by ice lemonade Heat lost by lemonade 63 Example 14. Getting Ready for a Party A 7.00-kg glass bowl [c = 840 J/(kg·C°)] contains 16.0 kg of punch at 25.0 °C. Two-and-a-half kilograms of ice [c = 2.00 × 103 J/(kg·C°)] are added to the punch. The ice has an initial temperature of –20.0 °C, having been kept in a very cold freezer. The punch may be treated as if it were water [c = 4186 J/(kg·C°)], and it may be assumed that there is no heat flow between the punch bowl and the external environment. The latent heat of fusion for water is 3.35 × 105 J/kg. When thermal equilibrium is reached, all the ice has melted, and the final temperature of the mixture is above 0 °C. Determine this temperature. 64 (a) (b) (c) 65 (d) (e) . 66 A dye-sublimation printer. As the plastic film passes in front of the print head, the heat from a given heating element causes one of three pigments or dyes on the film to sublime from a solid to a gas. The gaseous dye is absorbed onto the coated paper as a dot of color. The size of the dots on the paper has been exaggerated for clarity. 67 Check Your Understanding 4 When ice cubes are used to cool a drink, both their mass and temperature are important in how effective they are. The table below lists several possibilities for the mass and temperature of the ice cubes used to cool a particular drink. Rank the possibilities in descending order (best first), according to their cooling effectiveness. Mass of ice Temperature of cubes ice cubes c, a, b (a) m –6.0 °C (b) ½m –12 °C (c) 2m –3.0 °C 68 Equilibrium Between Phases of Matter The pressure of the vapor that coexists in equilibrium with the liquid is called the equilibrium vapor pressure of the liquid. 69 The equilibrium vapor pressure does not depend on the volume of space above the liquid. Only when the temperature and vapor pressure correspond to a point on the curved line, which is called the vapor pressure curve or the vaporization curve, do liquid and vapor phases coexist 70 in equilibrium. Conceptual Example 15. How to Boil Water That Is Cooling Down 71 Water is boiling in an open flask. Shortly after the flask is removed from the burner, the boiling stops. A cork is then placed in the neck of the flask to seal it. To restart the boiling, should you pour hot (but not boiling) water or cold water over the neck of the flask, as in part b of the drawing? It is possible to restart the boiling by pouring cold water over the neck of the flask. 72 The operation of spray cans is based on the equilibrium between a liquid and its vapor. 73 As is the case for liquid/vapor equilibrium, a solid can be in equilibrium with its liquid phase only at specific conditions of temperature and pressure. For each temperature, there is a single pressure at which the two phases can coexist in equilibrium. A plot of the equilibrium pressure versus equilibrium temperature is referred to as the fusion curve. 74 Humidity The partial pressure of a gas is the pressure it would exert if it alone occupied the entire volume at the same temperature as the mixture. When the partial pressure of the water vapor equals the equilibrium vapor pressure of water at a given temperature, the relative humidity is 100%. In such a situation, the vapor is said to be saturated because it is present in the maximum amount, as it would be above a pool of liquid at equilibrium in a closed container. 75 Example 16. Relative Humidities One day, the partial pressure of water vapor in the air is 2.0 × 103 Pa. Using the vaporization curve for water, determine the relative humidity if the temperature is (a) 32 °C and (b) 21 °C. 76 (a) (b) 77 When air containing a given amount of water vapor is cooled, a temperature is reached in which the partial pressure of the vapor equals the equilibrium vapor pressure. This temperature is known as the dew point. 78 79 Concepts & Calculations Example 17. Linear and Volume Thermal Expansion Three rectangular blocks are made from the same material. The initial dimensions of each are expressed as multiples of D, where D = 2.00 cm. They are heated and their temperatures increase by 35.0 C°. 80 The coefficients of linear and volume expansion are and respectively. Determine the change in their (a) vertical heights and (b) volumes. 81 (b) 82 Concepts & Calculations Example 18. Heat and Temperature Changes 83 Objects A and B are made from copper, but the mass of B is three times that of A. Object C is made from glass and has the same mass as B. The same amount of heat Q is supplied to each one: Q = 14 J. Determine the rise in temperature for each. 84 Conceptual Question 3 REASONING AND SOLUTION The plate is made of aluminum; the spherical ball is made of brass. The coefficient of linear expansion of aluminum is greater than the coefficient of linear expansion of brass. Therefore, if the plate and the ball are heated, both will expand; however, the diameter of the hole in the aluminum plate will expand more than the diameter of the brass ball. In order to prevent the ball from falling through the hole, the plate and the ball must be cooled. Both the diameter of the hole in the plate and the diameter of the ball will contract. The diameter of the hole will decrease more than the diameter of the ball, thereby preventing the ball from falling through the hole. 85 Problem 12 REASONING AND SOLUTION a. The radius of the hole will be larger when the plate is heated, because the hole expands as if it were made of copper. b. The expansion of the radius is r = r0 T . Using the value for the coefficient of thermal expansion of copper given in Table 12.1, we find that the fractional change in the radius is r/r0 = T = (17 * 10–6 C°–1)(110 °C 11 °C) 0.0017 86 Problem 18 REASONING AND SOLUTION The initial diameter of the sphere, ds, is ds = (5.0 * 10–4)dr + dr where dr is the initial diameter of the ring. Applying to the diameter of the sphere gives L L0 T d s s d s T and to the ring gives dr r dr T 87 If the sphere is just to fit inside the ring, we must have d s d s d r d r T d r ds sd s r d r Substituting Equation (1) in this result and taking values for the coefficients of thermal expansion of steel and lead from Table 10.1 yield 4 T 5.0 10 29 10 6 C 1 5.0 10 4 1 12 10 6 C 1 Tf = 70.0 °C - 29 C° = 29 C 41 C 88 Problem 30 REASONING AND SOLUTION Both the water and pipe expand as the temperature increases. VP CV0 T VW W V0 T The initial volume of the pipe and water is V0 r L 2 V VW VP ( W C )V0 T V (207 10 C 5110 C ) (9.5 10 m) (76m)(54C) 6 1 4 1.8 10 m 6 3 1 3 2 89 Problem 32 REASONING AND SOLUTION Both the coffee and beaker expand as the temperature increases. VC WV0 T Vb bV0 T V VC Vb ( w b )V0 T Taking the coefficients of volumetric expansion w and c for coffee (water) and glass (Pyrex) from Table 12.1, we find 6 1 6 1 3 V 207 10 (C) 9.9 10 (C) (0.5 10 m )(92C 18C ) =7.3*10-6m3 3 90 Problem 46 REASONING AND SOLUTION We wish to convert 2.0% of the heat Q into gravitational potential energy, i.e., (0.020)Q = mgh. Thus mg 0.020 Q h 4186 J 0.020 110 Calories 1 Calorie 2.1 m 4.4 10 3 N 91