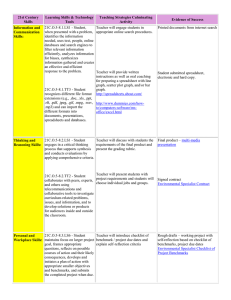
0 o C – (100 o C)
advertisement

KEY Practice Thermochemistry Test : 3.3-3.5, 9.5, 10.5 The list of equations for this chapter is attached to the back of the test. Tear them off and use them to help you answer the questions. “Show ALL Work!” Show ALL work to receive full credit CIRCLE final answers Include units with final answers Round all answers to the 100ths place Refer to the following Heating Curve for water as you answer Question #1. 1) i) (a) Calculate the energy required, in kilojoules, FOR EACH STEP to decrease the temperature of 20.0g of water from 120. oC to 0oC. CLEARLY show and IDENTIFY each step. Show ALL work, CIRCLE answers! Cool steam: q = msteam × csteam × ΔTsteam 𝐉 q = 20.0 g × 1.841 × (100oC – 120oC) ] 𝐠−𝐊 q = 36.82 ii) 𝐉 𝐊 × ─ 20 oC → q = ─ 736.4 J ÷ 1,000 = ─ 0.7364 kJ Condense steam: 𝒒𝒗𝒂𝒑 = mice × 𝚫𝑯𝒗𝒂𝒑𝑯 𝒒𝒇𝒖𝒔 = 20.0 g × 2,260 iii) 𝐉 𝐠 𝟐𝑶 = 45,200 J ÷ 1,000 = ─ 45.2 kJ Cool water: q = mwater × cwater × ΔTwater 𝐉 q = 20.0 g × 4.184 × [ 0oC – (100oC) ] 𝐠−𝐊 q = 83.68 𝐉 𝐊 × ─100 oC → q = ─ 8,368 J ÷ 1,000 = ─ 8.368 kJ released (b) During this process is energy being absorbed or released? __________________________ 1 2) In the old days, on a cold winter night it was common to bring a hot object to bed with you. Which of the following objects – a 10-kilogram iron brick or a 2-kilogram jug of hot water – would be better to warm up your bed to a nice, cozy temperature of 25.0oC ? Use calculations as part of your answer. Write a few sentences to justify your choice. Don't forget to: (a) clearly indicate WHICH SUBSTANCE would be the best choice (b) use CALCULATIONS to illustrate WHY this substance would be the best choice (c) write a few sentences in which you COMPARE the results of your calculations and HOW they justify your choice. Substance Water Iron Mass (kg) 2 10 Specific Heat J g Co 4.184 0.45 ΔT (oC) 25.0 25.0 (a) 2 kg of water would be the would be the best choice (b) calculations to determine the energy which could be released by each substance 𝒒𝑯𝟐 𝑶 𝒐𝒓 𝑭𝒆 = 𝒎𝑯𝟐 𝑶 𝒐𝒓 𝑭𝒆 × 𝒄𝑯𝟐 𝑶 𝒐𝒓 𝑭𝒆 × ΔT i) q = 2000 g × 4.184 J g Co ii) q = 10,000 g × 0.45 J g Co × 25.0oC → 209,200 J of energy for water × 25.0oC → 112,500 J of energy for iron (c) 2 kilograms of WATER would be the best choice to keep you warm because it can store more energy energy (209,200 J) than 10 kilograms of iron (112,500 J). The more energy it stores the longer it will be able to keep you warm 2 3) (a) What is heat (thermal energy) and how is it different from temperature? Heat = energy which is being transferred from one object to another due to a temperature difference Temperature = a measure of the average EK of an object (b) What is internal energy? the sum of the EK and EP of all of the atoms of a substance (c) How are heat and internal energy related? The more internal energy an object has the more “heat” it will have available to transfer BONUS: A 35.2-g sample of a metal heated to 100.0oC is placed in a calorimeter containing 42.5 g of water at an initial temperature of 19.2oC. If the final temperature of the metal and the water is 29.5oC, what is the specific heat of the solid? 1st: calculate ΔTwater ΔT = Tf ─ Ti → 29.5oC − 19.2oC = 10.3oC 2nd : calculate energy gained by water 𝐉 qwater = (42.5 g) (4.184 𝒈 × 𝑲 ) (10.3oC ) → 1812.29 J 3rd : remember that +qwater = ─qmetal ─qmetal = ─ 1812.29 J 4th : calculate cmetal ─ 1812.29 J = 35.2 g × cmetal × (29.5oC – 100oC) = 0.730 𝐉 𝒈×𝑲 3 Equation Sheet for Thermodynamics Test cice= 2.03 𝐉 𝚫𝑯𝒗𝒂𝒑𝑯 𝟐𝑶 𝐠−𝐊 cwater = 4.184 csteam = 1.841 𝐉 𝐠−𝐊 𝐉 𝐠−𝐊 Δ𝑯𝒇𝒖𝒔𝑯 𝟐 = = 𝑶 𝟐𝟐𝟔𝟎 𝐉 𝐠 𝟑𝟑𝟒 𝐉 𝐠 q = m × c × ΔT 𝒒𝒗𝒂𝒑 = m × Δ𝑯𝒗𝒂𝒑 𝒒𝒇𝒖𝒔 = 𝐦 × 𝚫𝑯𝒇𝒖𝒔 4 5