The Basics
Bonding and Molecular
Structure
Chapter 1
1.2 Atomic Structure
Compounds
made up of elements combined in different
proportions
Elements
made up of atoms
Atoms
positively charged nucleus containing
protons and neutrons
with a surrounding cloud of negatively
charged electrons
1.2 Atomic Structure
Each element is distinguished by its atomic number, Z
Atomic number = number of protons in nucleus
Isotopes: Atoms of the same element (same # of
protons) may have different masses because they have
different numbers of neutrons
12C
13C
14C
(6 protons
6 neutrons)
(6 protons
7 neutrons)
(6 protons
8 neutrons)
1.2 Atomic Structure
Electrons that surround the nucleus exist in shells of
increasing energy and at increasing distances from the
nucleus.
Valence Electrons
Electrons in the outermost shell an atom uses in making
chemical bonds with other atoms to form compounds
The number of electrons in the valence shell is equal to
the group number of the atom
1.3 Chemical Bonds
Ionic (or electrovalent) bonds are formed by the transfer
of one or more electrons from one atom to another to
create ions
Covalent bonds result when atoms share electrons
In forming compounds, they gain, lose, or share electrons
to give a stable electron configuration characterized by
a full shell of valence electrons
For the second row elements this is 8 electrons – octet rule
Electronegativity (EN)
The measure of the ability of an atom to attract electrons
Electronegativity is based on an arbitrary scale, with F the
most electronegative (4.0) and Cs the least (0.7)
1.3A Ionic Bonds
Atoms may gain or lose electrons and form charged particles
called ions
An ionic bond is an attractive force between oppositely
charged ions
Ionic bonds form between atoms with a large EN difference
Li 1s22s1
F 1s22s2p5
Li 1s2
F 1s22s2p6
1.3B Covalent Bonds
Molecules may also contain ions and covalent bonds:
H
H
N
H
H
(ammonia)
(3 bonds on N)
H+
H
N
H
H
(ammonium cation)
(4 bonds on N with
a positive charge on N)
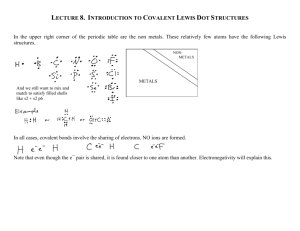
1.4 Lewis Structures
Lewis structures show the connections between atoms in a
molecule or ion using only the valence electrons of the atoms
involved
For main group elements, the number of valence electrons a
neutral atom brings to a Lewis structure is the same as its group
number
If the structure is an anion (negative ion) we add one electron for
each negative charge and if the structure is a cation (positive
ion), we subtract one electron for each positive charge
In drawing Lewis structures we try to give each atom the electron
configuration of a noble gas
1.4 Lewis Structures
Steps for Drawing Lewis Structures
1.
2.
3.
4.
5.
6.
7.
Find the total # of valence electrons for all atoms
Adjust the # if any negative or positive charges are indicated
Arrange atoms logically – large elements in middle,
hydrogens/halogens on the periphery
Connect the molecule with single bonds (2 e- each)
Assign remaining electrons to the most electronegative
elements first as lone pairs
Complete octets (if possible) with multiple bonds
Assign formal charges, if necessary
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Know Your Elements
H fills its shell with two electrons or zero (H+)
Neutral C, N, O, F always follow octet rule (and usually Cl, Br, I)
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Know Your Elements
Group IA Metals only form +1 cations
Group IIA Metals only form +2 cations
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Know Your Elements
Group IIIA may follow the octet rule or prefer a sextet of eThe higher halogens usually follow the octet rule, but may
expand their octet if they are the central element in the
molecule (F always follows the octet rule)
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Know Your Elements
The main group non-metals may follow the octet rule but very
often expand their octet
The transition metals can form ions, follow the octet rule or
expand their octets
1.5 Formal Charge
Formal charge is the charge assigned to individual atoms in a
Lewis structure.
Formal charge is calculated as follows:
• The number of electrons “owned” by an atom is determined by
its number of bonds and lone pairs.
• An atom “owns” all of its unshared electrons and half of its
shared electrons.
Know Your Lewis Structures
Once you practice enough Lewis structures you begin to see
that the elements we cover in this course follow a similar
pattern
Know Your Lewis Structures
Once you practice enough Lewis structures you begin to see
that the elements we cover in this course follow a similar
pattern
Know Your Lewis Structures
Once you practice enough Lewis structures you begin to see
that the elements we cover in this course follow a similar
pattern
5A. A Summary of Formal Charges
© 2014 by John Wiley & Sons, Inc. All rights reserved.